 |
SPORES single in the soil; develop laterally on the neck of a sporiferous saccule; yolk yellow (4B3) to light brown (6D8); globose to subglobose; (110-)140(-170) µm diam; sometimes irregular; sessile on the neck of a sporiferous saccule.
SUBCELLULAR STRUCTURE OF SPORES consists of a spore wall and two inner germination walls.
 |
||||||
In PVLG |
In Melzer's |
In PVLG |
||||
 |
||||||
In PVLG
|
In PVLG+Melzer's reagent |
|||||
In PVLG+Melzer's reagent |
|||
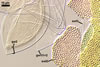
Spore wall contains three layers (swl 1-3).
Layer 1 evanescent, hyaline, (0.8-)1.3(-1.5) µm thick, continuous with the wall of a sporiferous saccule, usually completely sloughed in mature spores.
Layer 2 laminate, yolk yellow (4B8) to light brown (6D8), (5.1-)5.7(-6.6) µm thick, evenly pitted with round, 2-5 µm diam, rarely ovate, 2.0-2.9 x 4.2-5.0 µm and 1.7-2.5 µm deep depressions, separated by ridges, 1.0-4.0 µm wide.
Layer 3 flexible, hyaline, (1.2-)2.0(-3.4) µm thick, easily separating from layer 2.
Germination wall 1 composed of two, tightly adherent, semiflexible, hyaline layers (gw1l1 and 2), each 0.5-0.8 µm thick.
Germination wall 2 consists of three layers (gw2l1-3).
Layer 1 flexible, hyaline, up to 0.5 µm thick, evenly ornamented with small knobs; this layer usually completely tears and disappears in vigorously crushed spores.
Layer 2 flexible, coriaceous, hyaline, (1.5-)1.7(-2.9) µm thick.
Layer 3 plastic, hyaline, 3.1-8.0 µm thick in PVLG, 0.8-1.5 µm thick and beetroot purple (13D8) in Melzer’s reagent.
GERMINATION ORB. Not found.
SPORIFEROUS SACCULE hyaline to pale orange (5A3); globose to subglobose; 130-150 µm diam; neck 100-120 µm long, tapering from 30 µm diam at the saccule to 20 µm diam at the point of spore attachment. Saccule usually collapses or falls off in mature spores.
CICATRIX. A low collar, flat or slightly raised, circular to ovoid, 7.0-12.0 µm diam when observed in a plan view, with a centrally positioned pore.
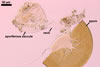
MYCORRHIZAE. Acaulospora cavernata has been associated in the field with vesicular-arbuscular mycorrhizal Salix triandra L. and Thuja occidentalis L. (Blaszkowski 1989). Many attempts to initiate sporulation of this fungus in both trap and one-species cultures failed.
DISTRIBUTION. The only known site of occurrence of Ac. cavernata is that from Hel (54o36’N, 18o49’E) where spores of this fungus occurred among mycorrhizal roots of S. triandra and T. occidentalis growing ca. 200 m from the Baltic Sea (Blaszkowski 1989, 1994).
NOTES. Acaulospora cavernata highly resembles Ac. foveata Trappe & Janos due to the formation of dark-coloured and pitted spores. However, spores of the latter species are much larger. Spores of Ac. cavernata do not reach even the lower range of diameter of Ac. foveata spores (Janos and Trappe 1982; Morton 2000). Additionally, differences between these fungi occur in the total number of layers present in the subcellular structure of their spores, as well as in the phenotypic and biochemical properties of these layers. In Ac. foveata, the total number of layers in the spore wall and the inner germination walls is seven, whereas eight layers occur in spores of Ac. cavernata. The third layer in the spore wall of Ac. foveata is coloured and stains dark red-brown in Melzer’s reagent (Morton 2000). In contrast, the third spore wall layer of Ac. cavernata is hyaline and nonreactive in this reagent.
The knobby layer 1 of the germination wall 2 is not homologous with a beaded, flexible layer present in germination wall 2 of Ac. foveata and most other members of Acaulospora because of differences in the position and nature of these layers. In Ac. cavernata, the knobby layer is the outermost one in the 3-layered germination wall 2 and is associated with a flexible layer, non-reactive in Melzer’s reagent. In contrast, a beaded, flexible layer of Ac. foveata and most other Acaulospora spp. always adheres to an innermost layer staining darkly in Melzer’s reagent. The knobby layer of A. cavernata resembles a plastic covering of the inner surface of post envelopes.
REFERENCES
Blaszkowski J. 1989. Acaulospora cavernata (Endogonaceae) - a new species from Poland with pitted spores. Crypt. Bot. 1, 204-207.
Blaszkowski J. 1994. Arbuscular fungi and mycorrhizae (Glomales) of the Hel Peninsula, Poland. Mycorrhiza 5, 71-88.
Janos D. P., Trappe J. M. 1982. Two new Acaulospora species from tropical America. Mycotaxon 15, 515-522.
Morton J. B. 2000. International Culture Collection of Arbuscular and Vesicular-Arbuscular Mycorrhizal Fungi. West Virginia University.