O
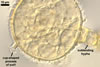
n potato glucose agar
medium, C. moniliformis formed slow-growing, caramel (6C6) to rust
brown (6E8), low, downy cultures with an irregular margin. After 35 days,
these cultures reached 60-70 mm diam and started to form spores. The
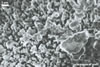
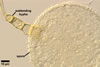
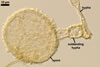
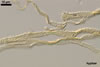
hyphae from the agar medium were hyaline to pale yellow (3A3), verrucose,
5-7.5 µm diam, divided by septa; the distance between the septa ranged
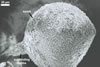
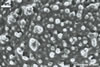
from 22.5 to 50 µm. The spores were hyaline to pale yellow (3A3), globose
to subglobose, 30-70 µm diam, and had one 2-layered wall, 1-5 µm
thick. This wall was ornamented with warts, spines, finger- or cup-shaped
processes, up to 10 µm high. The subtending hypha was cylindrical, flared
or constricted, hyaline to pale yellow (3A3), 10-20 µm wide at the spore
base and 20-130 µm long, with 2-11 septa.
MYCORRHIZAE.
Generally, fungi of the genus Complexipes
are considered to occur in associations with ectendomycorrhizae (Laiho 1965;
Mikola 1965; Thomas and Jackson 1982; Wilcox et al. 1974), although Wilcox
et al. (1974) insisted that some of them produce ectendomycorrhizae with
a given pine while others form ectomycorrhizae in the same pine. The species
of pine is also important whether the association will be ecto- or ectendomycorrhizal.
The function of the
fungi in mycorrhizal associations is poorly known. Pachlewski (1983) isolated
a fungus called MrgX from ectendomycorrhizae of Pinus sylvestris L.,
which seems to be of the genus Complexipes. In his laboratory and
field studies, the MrgX isolates were highly active mycorrhizal fungi forming
a biotic barrier for ectomycorrhizal symbionts of pine. Danielson et al.
(1984a-d) also classified fungi of Complexipes to the most effective
mycorrhizal fungi.
DISTRIBUTION.
The
fungi of the genus Complexipes are widely distributed on coniferous
and deciduous hosts in tree nurseries, sand dunes, burned sites, and disturbed
areas in Canada (Danielson 1991; Danielson et al. 1984a-d), the United States
(Laiho 1965; Wilcox et al. 1974, 1983; Yang and Wilcox 1984), the United
Kingdom (Thomas et al. 1983), Finland (Mikola 1965), Poland (Blaszkowski
1989), Kenya (Ivory and Pearce 1991), and New Zealand and Australia (Mosse
and Bowen 1968).
REFERENCES
Blaszkowski J. 1989.
The occurrence and geographic distribution of E-strain ectendomycorrhizal
fungi in Poland. Bull. Pol. Ac. Sci. Biol. Sci. 37, 19-31.
Danielson R. M. 1991.
Temporal changes and effects of amendments on the occurrence of sheathing
(ecto-) mycorrhizas of conifers growing in oil sands tailings and coal spoil.
Agric. Ecosyst. Environ. 35, 261-281.
Danielson R. M., Griffiths
C. L., Parkinson D. 1984a. Effects of fertilization on the growth and mycorrhizal
development of container grown pine seedlings. Forest Sci. 30, 828-835.
Danielson R. M., Visser
S., Parkinson D. 1984b. Microbial activity and mycorrhizal potential of
four overburden types used in the reclamation of extracted oil sands. Can.
J. Soil Sci. 63, 363-375.
Danielson R. M., Visser
S., Parkinson D. 1984c. Production of ectomycorrhizae on container-grown
jack pine seedlings. J. For. Res. 14, 33-36.
Danielson R. M., Zak
J. C., Parkinson D. 1984d. Mycorrhizal inoculum in a peat deposit formed
under a white spruce stand in Alberta. Can. J. Bot. 63, 2557-2560.
Ivory M. H., Pearce
R. B. 1991. Wilcoxina mikolae newly identified as a mycorrhizal
fungus on pines in Africa. Mycol. Res. 95, 250-253.
Laiho O. 1965. Further
studies on the ectendotrophic mycorrhiza. Acta For. Fenn. 79, 1-34.
Mikola P. 1965. Studies
on the ectendotrophic mycorrhiza of pine. Acta For. Fenn. 75, 1-56.
Mosse B., Bowen G.
D. 1968. A key to the recognition of some Endogone spore types.
Trans. Br. Mycol. Soc. 51, 469-483.
Pachlewski R. 1983.
Grzyby symbiotyczne i mikoryzy sosny (Pinus silvestris L.). Prace
IBL 615, 3-123.
Thomas G. W., Jackson
R. M. 1982. Scanning electron microscopy of sheathing mycorrhizas of Sitka
spruce. Trans. Br. Mycol. Soc. 79, 31-39.
Thomas G. W., Rogers
D., Jackson R. M. 1983. Changes in the mycorrhizal status of Sitka spruce
following outplanting. Plant Soil 71, 319-323.
Wilcox H. E., Ganmore-Neumann
R., Wang C. J. K. 1974. Characteristics of two fungi producing ectendomycorrhizae
in Pinus resinosa. Can. J. Bot. 52, 2279-2282.
Wilcox, H. E., Yang
C. S., LoBuglio K. 1983. Responses of pine to E-strain ectendomycorrhizal
fungi. Plant and Soil 71, 239-247.
Yang C. S., Wilcox
H. E. 1984. An E-strain ectendomycorrhiza form by a new species, Tricharina
mikolae. Mycologia 76, 675-684.