 |
 |
 |
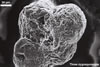
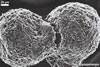
SPOROCARPS ovoid; 170-360 x 240-510 µm; containing 2-7 randomly positioned zygosporangia enveloped individually by a hyphal mantle.
 |
 |
 |
SPOROCARPS ovoid; 170-360 x 240-510 µm; containing 2-7 randomly positioned zygosporangia enveloped individually by a hyphal mantle.
Peridium absent.
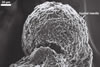
Mantle (15.0-)18.6(-22.0) µm thick; consisting of interwoven, hyaline hyphae; (2.0-)4.6(-8.3) µm diam; usually staining lake red (9C8) in Melzer’s reagent, although hyphae of the outermost zone, forming the mantle surface, sometimes remain unstained or stain pinkish white (8A2). Red reaction of different intensity of the hyphal mantle in Melzer’s reagent has also been recorded in E. alba (Petch) Gerd. & Trappe (Yao et al. 1992), E. pseudopisiformis Y. J. Yao (Yao et al. 1995b), and Youngiomyces carolinensis Y. J. Yao (Yao et al. 1995a). Lighter staining of the outer than the inner hyphae of a mantle also occurs in E. pseudopisiformis (Yao et al. 1995b).
The inner surface of a hyphal mantle is formed by agglutinated hyphae producing a sheath adherent to the zygosporangium. This sheath may vary from thin to relatively thick and frequently separates from the zygosporangial wall. The sheath bears more loosely interwoven hyphae, frequently branched or convoluted in the outer zone of the mantle. In a cross-sectional view, the mantle consists of several tightly adherent hyphal layers. When observed in a plane view, the mantle resembles a cerebrum.
 |
 |
 |
In PVLG |
||
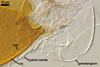
Zygosporangial wall (zlw) consisting of a smooth, sunflower yellow (4A7) to brownish yellow (5C8) layer, (1.7-)2.0(-2.2) µm thick.
Zygospore wall (zew) single, smooth, hyaline, (2.0-)2.9(-5.6) µm thick, separable from the zygosporangial wall. In crushed specimens, the zygospore wall usually easily separates from the zygosporangial wall, and the whole zygospore frequently releases the zygosporangium. The zygospore stains orange white (6A2) in Melzer’s reagent, a property not reported in the literature so far (Pegler et al. 1993; Trappe and Gerdemann 1972).
 |
 |
In PVLG |
|
Gametangial wall smooth, (1.2-)4.3(-6.9) µm thick at the zygosporangial base; wall of the larger gametangium continuous with the zygosporangial wall; gametangia usually covered by the hyphal mantle of zygosporangia.
Zygospore contents of hyaline lipid globules. Zygospores staining orange white (6A2) in Melzer’s reagent.
Zygosporangia and gametangia nonreactive in Melzer’s reagent.
DISTRIBUTION. See above. No report of the presence of this fungus in other regions of the world exists.
NOTES. Endogone maritima is distinctive due to its zygosporangia grouped in small sporocarps lacking a peridium or occurring singly in the soil and in the nonreactivity of the zygosporangial wall in Melzer’s reagent. The sporocarps are compact and usually contain 2-3 zygosporangia bound by interwoven hyphae coming from the mantle surrounding each individual zygosporangium.
Despite the fungi of the genus Endogone are considered to form zygosporangia in compact sporocarps (Gerdemann and Trappe 1974; Pegler et al. 1993), the author of this website also found specimens of E. aurantiaca Blaszk., E. flammicorona Trappe & Gerd., and E. lactiflua Berk. & Broom (Blaszkowski 1989, 1993, 1997) to produce single zygosporangia and those arranged in small aggregates (see the discussion in the section “the Endogonaceae”).
The formation of small sporocarps by E. maritima may result from the exceptionally unstable character and the physical and chemical properties of the soils in which this fungus occurred. These soils are represented by highly mobile sand dunes low in organic matter. In contrast, the literature indicates that other Endogone spp. producing large sporocarps occur in stable sites with relatively high organic matter content. Endogone maritima is the first species of this genus recovered from maritime dunes.
The gametangia of the E. maritima specimens usually are covered with a hyphal mantle and, therefore, are poorly visible. One of the two gametangia associated with each zygosporangium is larger with a thicker wall and is connected with a pore in the zygosporangial wall. The second gametangium adheres to the larger and seems to have no contact with the zygosporangium. Thus, E. maritima zygosporangia probably bud from the tip of the larger of the two gametangia, similarly as those of E. flammicorona and E. lactiflua (Trappe and Gerdemann 1972).
Zygosporangia of E. maritima superficially resemble those of E. lactiflua. The two fungi form zygosporangia surrounded by a hyphal mantle appearing netted in a cross-sectional view. However, the differences in size of sporocarps, the number of zygosporangia grouped in them, shape and color of zygosporangia, breadth of gametangia, as well as in reaction of the zygosporangial and zygospore walls in Melzer’s reagent revealed based on both literature data (Gerdemann and Trappe 1974; Pegler et al. 1993) and personal observations of the author of this website readily separate the two fungal species. Endogone maritima compared with E. lactiflua produces much smaller sporocarps (150-370 µm vs. 0.5-20 µm) with much lower number of zygosporangia (2-7 vs. hundreds). The zygosporangia of E. maritima mostly are globose to subglobose and sunflower yellow to brownish yellow, whereas those of E. lactiflua usually are broader than long and brown. In contrast to the nonreactive zygosporangial wall of E. maritima in Melzer’s reagent, that of E. lactiflua stains crayfish red (9B8) in this reagent.
Other Endogone spp. producing sporocarps lacking a peridium are E. acrogena Gerd., Trappe & Hosford and E. verrucosa Gerd. & Trappe (Gerdemann and Trappe 1974). However, E. acrogena has zygosporangia arranged in chains (vs. in ovoid sporocarps in E. maritima), and those of E. verrucosa are aggregated in clusters enclosed in an endoperidium, a phenomenon unknown in E. maritima.
The occurrence of E. maritima at sites dominated by plants associated with arbuscular mycorrhizal fungi suggests that this fungus is a saprophyte. Of the 13 so far known Endogone spp., only E. flammicorona, E. lactiflua, and E. tuberculosa Lloyd are known to form ectomycorrhizae (Trappe and Gerdemann 1972; Walker 1985; Warcup 1990).
REFERENCES
Blaszkowski J. 1989. The occurrence and geographic distribution of E-strain ectendomycorrhizal fungi in Poland. Bull. Pol. Acad. Sci. Biol. Sci. 37, 19-31.
Blaszkowski J. 1993. The occurrence of arbuscular fungi and mycorrhizae (Glomales) in plant communities of maritime dunes and shores of Poland. Bull. Pol. Ac. Sci. Biol. Sci. 41, 377-392.
Blaszkowski J. 1997. Endogone aurantiaca, a new species in the Endogonales from Poland. Mycotaxon 63, 131-141.
Blaszkowski J., Tadych M., Madej T. 1998. Endogone maritima, a new species in the Endogonales from Poland. Mycol. Res. 102, 1096-1100.
Gerdemann J. W., Trappe J. M. 1974. The Endogonaceae in the Pacific Northwest. Mycologia Memoir 5, 1-76.
Pegler D. N., Spooner B. M., Young T. W. K. 1993. British truffles. A revision of British hypogeous fungi. Royal Botanic Gardens, Kew. 216 pp. + 26 plates.
Trappe J. M., Gerdemann J. W. 1972. Endogone flammicorona sp. nov., a distinctive segregate from Endogone lactiflua. Trans. Br. Mycol. Soc. 59, 403-407.
Walker C. 1985. Endogone lactiflua forming ectomycorrhizas with Pinus contorta. Trans. Br. Mycol. Soc. 84, 353-355.
Warcup J. H. 1990. Taxonomy, culture and mycorrhizal associations of some zygosporic Endogonaceae. Mycol. Res. 94, 173-178.
Yao Y-J., Pegler D. N., Young T. W. K. 1992. Revision of the type of Endogone alba (Endogonaceae). Mycotaxon 45, 109-122.
Yao Y-J., Pegler D. N., Young T. W. K. 1995a. Youngiomyces, a new genus in Endogonales (Zygomycotina). Kew Bull. 50, 349-357.
Yao Y-J., Pegler D. N., Young T. W. K. 1995b. New species in Endogone (Endogonales). Kew Bull. 50, 359-365.