GERMINATION.
Not observed.
MYCORRHIZAE.
In the field, spores of E. baltica have been associated
with vesicular-arbuscular mycorrhizal roots of Ammophila
arenaria (L.) Link,
Elymus arenarius L., Helichrysum arenarium (L.) Moench,
Hieracium umbellatum L., Lathyrus japonicus subsp. maritimus
(L.) P. W. Ball., and Petasites
spurius (Retz.) Rchb.
(Blaszkowski
et al. 1998; Tadych
and Blaszkowski 2000). Although
E. baltica produced spores in trap cultures, many attempts to establish
mycorrhizae of this fungus in one-species cultures failed. No literature data
exists of the properties of mycorrhizae of E. baltica.
DISTRIBUTION.
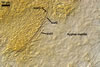
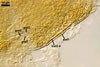
Entrophospora
baltica has originally been recovered from among roots of A. arenaria
and P. spurius colonizing dunes adjacent to Swinoujscie (53º55'N,
14º14'E) in north-western Poland (Blaszkowski et al. 1998). Later, this fungus
has been found associated with different plant species (see above) growing
in maritime dunes of the Slowinski National Park (54º45’N, 17º26’E;
Tadych and Blaszkowski 2000).
Apart from Poland, E. baltica has also been revealed at several Alpine elevations in Switzerland and among the mycorrhizal community of forest ecosystems near Valdina in Southern Chile (Sieverding and Oehl 2006).
NOTES.
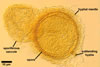
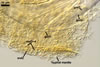
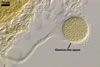
Spores of E. baltica are distinctive because of both being enveloped
in a sinuate hyphal mantle and having a unique wall structure. The mantle
consists of tightly interwoven thin-walled hyphae that evenly cover the spores
and sometimes occur on the sporiferous saccule. The presence of short hyphae
on the surface of immature spores and the lack of their evanescent outermost
wall layer 1 in crushed spores with a separated mantle suggest this mantle
to be formed by hyphae developing from spore wall layer 1. Spore wall layer
1 is visible in most intact spores. Spore wall layer 2 is always present and
sometimes detaches from spore wall layer 3 in uncrushed spores mounted in
lactic acid. Spore wall layer 3 consists of two to three laminae usually separating
from each other in vigorously crushed spores.
Spore wall layer 4 has not been included in the original description of the species (Blaszkowski et al. 1998), but it has been reported to exist by Sieverding and Oehl (2006). Germinal wall layer 1 is
a typical membranous wall. The coriaceous layer 2 of this wall is flexible
and does not crack, despite it is much thicker than the flexible germinal
wall layer 1. Germinal wall layer 3 has not been presented in the original protologue (Blaszkowski et al. 1998).
Entrophospora baltica
differs greatly from other species of the Glomeromycota forming entrophosporioid spores (Intraspora and Kuklospora spp.)
in appearance, the morphology of the spore ornamentation, and in spore wall
structure. None of the species forms spores enveloped
in a hyphal mantle. Entrophospora
infrequens, now the only other member of the genus Entrophospora, and Kuklospora kentinensis
produce spores with an ornamented laminate spore wall
layer. However, the ornamentation of the former fungus consists of vacuolated
projections
(Ames and Schneider 1979; Blaszkowski 1989; Hall 1977; Sieverding and Oehl 2006), and that of the latter
species is composed of evenly distributed pits (Morton 2000; Sieverding and Oehl 2006; Wu et al. 1995).
In contrast, spores of E. baltica are ornamented with warts present
on a permanent, unit spore wall layer adherent to a laminate layer. Kuklospora colombiana and Intraspora schenckii
may be separated readily from E. baltica
because of having smooth spores of a different subcellular structure (Schenck
et al. 1984; Sieverding and Oehl 2006; Sieverding and Toro 1987). Additionally, spores of E. schenckii
are hyaline (Sieverding and Toro 1987; Schenck et al. 1984), whereas those
of E. baltica are coloured.
Arbuscular fungi having
spores enveloped in a sinuate hyphal mantle also are Glomus mortonii, Gl.
sinuosum,
and Gl. tortuosum (Almeida and Schenck 1990; Bentivenga and Hetrick 1991; Schenck
and Smith 1982). However, the mantle of Gl. mortonii and Gl.
sinuosum consists of thick-walled hyphae, not reacting in Melzer's reagent. The
mantle hyphae of E. baltica are thin-walled and stain lake red
in this reagent. Although the mantle of Gl. tortuosum is composed
of thin-walled hyphae, this fungus produces spores with a spore wall composed
of only one laminate layer (Morton 2000). Spores of Glomus spp.
originate terminally from subtending hyphae (Morton and Benny 1990). Additionally, Gl.
mortonii and Gl. sinuosum produce spores in sporocarps. In
contrast,
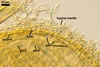
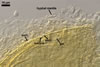
E. baltica spores develop within the neck of a sporiferous saccule
and occur singly in the soil.
REFERENCES
Almeida R. T., and N.
C. Schenck. 1990. A revision of the genus Sclerocystis (Glomaceae,
Glomales). Mycologia 82, 703-714.
Ames R. N., Schneider
R. W. 1979. Entrophospora, a new genus in the Endogonaceae. Mycotaxon
8, 347-352.
Bentivenga S. P., and
Hetrick B. A. D. 1991. Glomus mortonii sp. nov., a previously undescribed
species in the Glomaceae isolated from the tallgrass prairie in Kansas. Mycotaxon
42, 9-15.
Blaszkowski J. 1989.
Polish Endogonaceae. I. Acaulospora bireticulata, Entrophospora
infrequens, Glomus caledonium, and Scutellispora pellucida.
Karstenia 29, 1-10.
Blaszkowski J., Madej
T., Tadych M. 1998. Entrophospora baltica sp. nov. and Glomus
fuegianum, two species in the Glomales from Poland. Mycotaxon 68, 165-184.
Morton J. B. 2000. International
Culture Collection of Arbuscular and Vesicular-Arbuscular Mycorrhizal Fungi.
West Virginia University. http://www.invam.caf.wvu.edu/.
Morton J. B., Benny
G. L. 1990. Revised classification of arbuscular mycorrhizal fungi (Zygomycetes):
a new order, Glomales, two new suborders, Glomineae and Gigasporineae, and
two new families, Acaulosporaceae and Gigasporaceae, with an emendation of
Glomaceae. Mycotaxon 37, 471-491.
Hall I. R. 1977. Species
and mycorrhizal infections of New Zealand Endogonaceae. Trans. Br. Mycol.
Soc. 68, 341-356.
Schenck N. C., Smith
G. S. 1982. Additional new and unreported species of mycorrhizal fungi (Endogonaceae)
from Florida. Mycologia 74, 77-92.
Schenck N. C., Spain
J. L., Howeler R. H. 1984. Several new and unreported vesicular-arbuscular
mycorrhizal fungi (Endogonaceae) from Colombia. Mycologia 76, 685-699.
Sieverding E., Oehl F. 2006. Revision of Entrophospora and description of Kuklospora and Intraspora, two new genera in the arbuscular mycorrhizal Glomeromycetes. J. Appl. Bot. Food Qual. 80, 69-81.
Sieverding E., Toro
S. T. 1987. Entrophospora schenckii: a new species in the Endogonaceae
from Colombia. Mycotaxon 28, 209-214.
Tadych M., Blaszkowski
J. 2000. Arbuscular fungi and mycorrhizae (Glomales) of the Slowinski National
Park, Poland. Mycotaxon 74, 463-483.
Wu C.-G., Liu Y.-S.,
Hwuang Y.-L., Wang Y.-P., Chao C.-C. 1995. Glomales of Taiwan: V. Glomus
chimonobambusae and Entrophospora kentinensis, spp. nov.
Mycotaxon 53, 283-294.