|
In PVLG |
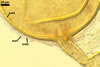
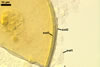
GERMINATION.
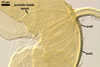
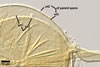
A germ tube emerges from the lumen of the subtending hypha.
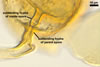
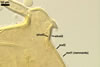
MYCORRHIZAE.
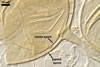
In roots of Plantago lanceolata L., mycorrhizae
of Gl. aggregatum consisted of arbuscules, intra- and abundant extraradical
hyphae staining moderately to intensively in 0.1% trypan blue. No vesicles were
found.
DISTRIBUTION. In
Poland, Gl. aggregatum has been found among roots of many plants
colonizing the Baltic Sea coast (Blaszkowski 1991, 1993a, 1994, 1995; Blaszkowski
et al. 2002a; Tadych and Blaszkowski 2000a) and sandy soils of the banks of
the Odra river (Blaszkowski 1991). It has been commonly associated with cultivated
plants of Poland (Blaszkowski 1993b). This fungus has been the third species
in frequency of occurrence in soils of the Bledowska Desert (50o22’N,
19o34’E; Blaszkowski et al. 2002b). Additionally, Gl. aggregatum
spores have been encountered in soils of the Tuchola Forests (53o46’N,
17o42’E-53o40’N, 17o54’E; Tadych and Blaszkowski 2000b).
Glomus aggregatum
has regularly occurred in dunes of the eastern coast of North America (Dalpé
1989; Friese and Koske 1991; Gemma and Koske 1989; Koske 1985, 1987; Sylvia
1986; Sylvia and Will 1988), Wisconsin (Koske and Tews 1987), San Miguel Island
(Halvorson and Koske 1987; Koske and Halvorson 1989), and Hawaii (Koske 1988).
NOTES.
Glomus aggregatum is most similar to Gl.
intraradices N.C. Schenck & G.S. Sm. and Gl. hoi S.M. Berch & Trappe.
All the species produce yellow-coloured spores of a similar size range and
the spores may occur both singly and in aggregates in the soil (Berch and
Trappe 1985; Blaszkowski 1991; Koske 1985; Schenck and Smith 1982). However,
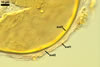
only Gl. aggregatum forms spores inside its parent spores by internal
proliferation.
REFERENCES
Berch S. M., Trappe J.
M. 1985. A new species of Endogonaceae, Glomus hoi. Mycologia 77,
654-657.
Blaszkowski J. 1991.
Polish Endogonaceae. IX. Glomus aggregatum with spores forming an
evanescent outermost wall. Crypt. Bot. 2/3, 130-135.
Blaszkowski
J. 1993a. The occurrence of arbuscular fungi and mycorrhizae (Glomales) in
plant communities of maritime dunes and shores of Poland. Bull. Pol. Ac. Sci.
Biol. Sci. 41, 377-392.
Blaszkowski J. 1993b.
Comparative studies of the occurrence of arbuscular fungi and mycorrhizae
(Glomales) in cultivated and uncultivated soils of Poland. Acta Mycol. 28,
93-140.
Blaszkowski J. 1994.
Arbuscular fungi and mycorrhizae (Glomales) of the Hel Peninsula, Poland.
Mycorrhiza 5, 71-88.
Blaszkowski J. 1995.
Glomus corymbiforme, a new species in Glomales from Poland. Mycologia
87, 732-737.
Blaszkowski J., Adamska
I., Czerniawska B. 2002a. Arbuscular mycorrhizal fungi (Glomeromycota) of
the Vistula Bar. Acta Mycol. 37, 39-62.
Blaszkowski J., Tadych
M., Madej T. 2002b. Arbuscular mycorrhizal fungi (Glomales, Zygomycota) of
the Bledowska Desert, Poland. Acta Soc. Bot. Pol. 71, 71-85.
Dalpé Y. 1989.
Inventaire et repartition de la flore endomycorhizienne de dunes et de rivages
maritimes du Québec, du Nouveau-Brunswick et de la Nouvelle-Ecosse.
Naturaliste Can. (Rev. Ecol. Syst.) 116, 219-236.
Friese C. F., Koske R.
E. 1991. The spatial dispersion of spores of vesicular-arbuscular mycorrhizal
fungi in a sand dune: microscale patterns associated with the root architecture
of American beachgrass. Mycol. Res. 95, 952-957.
Gemma J. N., Koske R.
E. 1989. Field inoculation of American beachgrass (Ammophila breviligulata)
with V-A mycorrhizal fungi. J. Environm. Manag. 29, 173-182.
Halvorson W. L., Koske
R. E. 1987. Mycorrhizae associated with an invasion of Erechtites glomerata
(Asteraceae) on San Miguel Island, California. Madrono 34, 260-268.
Koske R. E. 1985. Glomus
aggregatum emended: A distinct taxon in the Glomus fasciculatum complex.
Mycologia 77, 619-630.
Koske R. E. 1987. Distribution
of VA mycorrhizal fungi along a latitudinal temperature gradient. Mycologia
79, 55-68.
Koske R. E. 1988. Vesicular-arbuscular
mycorrhizae of some Hawaiian dune plants. Pacific Sci. 42, 217-229.
Koske R. E., Halvorson
W. L. 1989. Mycorrhizal associations of selected plant species from San Miguel
Island, Channel Islands National Park, California. Pacific Sci. 43, 32-40.
Koske R. E., Tews L.
L. 1987. Vesicular-arbuscular mycorrhizal fungi of Wisconsin sandy soils.
Mycologia 79, 901-905.
Schenck N. C., Smith
G. S. 1982. Additional new and unreported species of mycorrhizal fungi (Endogonaceae)
from Florida. Mycologia 74, 77-92.
Sylvia D. M. 1986. Spatial
and temporal distribution of vesicular-arbuscular mycorrhizal fungi associated
with Uniola paniculata in Florida foredunes. Mycologia 78, 728-734.
Sylvia D. M., Will M.
E. 1988. Establishment of vesicular-arbuscular mycorrhizal fungi and other
microorganisms on a beach replenishment site in Florida. Appl. Environm. Microbiol.
54, 348-352.
Tadych M., Blaszkowski
J. 2000a. Arbuscular fungi and mycorrhizae (Glomales) of the Slowinski National
Park, Poland. Mycotaxon 74, 463-483.
Tadych M., Blaszkowski
J. 2000b. Arbuscular mycorrhizal fungi of the Brda river valley in the Tuchola
Forests. Acta Mycol. 35, 3-23.