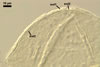
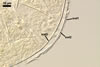
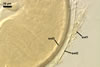
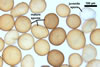
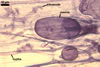
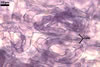
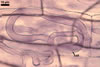
Layer
1 evanescent, hyaline, (0.5-)0.9(-1.5) µm thick before
disintegration, closely adherent to layer 2, smooth in juvenile spores,
gradually deteriorating and sloughing at the end of formation of layer 2,
always absent in mature spores.
Layer
2 flexible to semiflexible (folding when separated from
the laminate layer 3), hyaline, smooth, (0.7-)1.1(-1.5) µm thick,
sloughing with age, rarely present in mature spores.
Layers 1 and 2 are
continuous with a two-layered subtending hypha of juvenile spores.
Layer
3 laminate, smooth, orange (5B8) to raw umber (5F8), (2.0-)6.1(-8.8)
µm thick in mature spores, formed by gradual synthesis of very thin,
approximately 0.5 µm thick, laminae in the spore and its subtending
hypha; the first lamina is highly flexible and frequently separates from
layer 2 in crushed spores.
Spore wall layers 1-3
not reacting in Melzer’s reagent.
Layers 1 and 2, when
not deteriorated, swell in lactic acid-based mountants. Layer 3 then appears
to be covered with blisters or surrounded with an aureola.
MYCORRHIZAE.
In the field, Gl. arenarium has been associated with vesicular-arbuscular
mycorrhizal roots of Ammophila
arenaria (L.) Link, Artemisia campestris L., Helichrysum
arenarium (L.) Moench., Petasites spurius (Retz.) Rchb.,
and Senecio sp.
(Blaszkowski et al. 2001, 2002a, b; Tadych and Blaszkowski 2000).
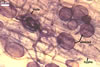
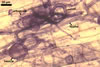
The mycorrhizae formed
in single-species cultures of this fungal species with Plantago lanceolata
L. and Zea mays L. as the hosts were composed of
arbuscules,
vesicles, and hyphae (Blaszkowski et al. 2001; Blaszkowski, pers. observ.).
Arbuscules were numerous and had fine branches difficult to see clearly. Vesicles
were ellipsoid, 45-80 x 50-100 µm. Hyphae were (3.7-)6.1(-8.0) µm
wide and grew parallel to the root axis. They were straight or slightly curved,
sometimes dichotomously branched and frequently coiled. These coils were 18.6-27.3
x 29.7-37.7 µm and evenly distributed along the root fragments examined.
In 0.1% trypan blue, arbuscules stained violet white (17A2) to pale violet
(17A3), vesicles
violet (18A3) to bluish violet (18A7), and intraradical hyphae bluish
white (23A2) to pastel violet (17A4).
DISTRIBUTION.
In
Poland, the sites found to harbour Gl. arenarium were maritime dunes
adjacent to Swinoujscie (53º55’N, 14º14’E; Blaszkowski et al.
2001), those of the Slowinski National Park (54º45’N,17º26’E;
Tadych and Blaszkowski 2000), the Vistula Bar (54º24'N,
19º30'E; Blaszkowski et al. 2002a),
and inland dunes of the Bledowska Desert (50º22’N, 19º34’E; Blaszkowski
et al. 2002b).
Blaszkowski et al. (2001) found spores of Gl. arenarium in maritime
sand dunes adjacent to Tel Aviv, Israel. No
data exist of the presence of this fungus in other regions of the world.
NOTES.
The only described species of the genus Glomus resembling Gl.
arenarium is Gl. etunicatum W.N. Becker & Gerd. The two fungi form spores similar
in colour, size and shape with a narrow subtending hypha, whose wall is much
lighter-coloured than the wall of its spore. Examination of the spore wall
structure easily separates these fungi. The spore wall of Gl. etunicatum
consists of a mucilaginous outer layer tightly adherent to a laminate inner
layer (Stürmer and Morton 1997). In contrast, the wall of Gl. arenarium
spores is composed of a sloughing outermost layer closely associated with
a semiflexible middle layer that easily separates from a laminate innermost
layer. Although the outermost layers of the two fungi are similar in appearance
and quickly slough, the mucilaginous layer of Gl. etunicatum spores
stains dark pinkish to reddish purple and that of spores of Gl. arenarium
does not react in this reagent. Additionally, the subtending hypha of Gl.
arenarium spores is persistent and almost always present in mature spores,
whereas that of spores of Gl. etunicatum frequently breaks at the
spore base, causing the spores to be similar to those of Acaulospora
and Entrophospora spp. when observed at a low magnification.
REFERENCES
Blaszkowski J., Tadych
M., Madej T. 2001. Glomus arenarium, a new species in Glomales (Zygomycetes).
Acta Soc. Bot. Pol. 70, 97-101.
Blaszkowski J., Adamska
I., Czerniawska B. 2002a. Arbuscular mycorrhizal fungi (Glomeromycota) of
the Vistula Bar. Acta Mycol. 37, 39-62.
Blaszkowski J., Tadych
M., Madej T. 2002b. Arbuscular mycorrhizal fungi (Glomales, Zygomycota) of
the Bledowska Desert, Poland. Acta. Soc. Bot. Pol. 71, 71-85.
Blaszkowski J., Tadych
M., Madej T., Adamska I., Iwaniuk A. 2001. Arbuscular mycorrhizal fungi (Glomales,
Zygomycota) of Israeli soils. Mat. II Polsko-Izraelskiej Konf. Nauk. nt. „Gospodarowanie
zasobami wodnymi i nawadnianie roslin uprawnych”. Przeglad naukowy Wydz.
Inz. Ksztalt. Srod. 22, 8-27.
Stürmer
S. L., Morton J. B. 1997. Developmental patterns defining morphological characters
in spores of four species in Glomus. Mycologia 89, 72-81.
Tadych M., Blaszkowski
J. 2000. Arbuscular fungi and mycorrhizae (Glomales) of the Slowinski National
Park, Poland. Mycotaxon 74, 463-483.