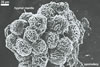
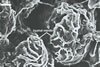
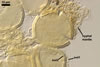
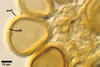
GERMINATION.
Not observed.
MYCORRHIZAE.
The ability of Gl. fuegianum to form mycorrhizae has not been experimentally
confirmed so far. The type specimen of Gl. fuegianum has been found
under moss (Pegler et al. 1993). Although Godfrey (1957) found this fungus
under Taxus baccata L., the other reports from the United Kingdom
informed of the occurrence of Gl. fuegianum associated with above-ground
plant parts, including living or dead culms of Eriophorum angustifolium
Honck., Molinia caerulea (L.) Moench, Nardus stricta L.,
Polytrichum and Sphagnum, as well as with rootstock of Juncus
effusus L. and wet debris of a Molinia sp.
In Poland, Gl. fuegianum
has been found in the field among vesicular-arbuscular mycorrhizal roots of
Juniperus communis L. (Blaszkowski et al. 1998). Although Gl.
fuegianum formed new spore-groups in trap cultures with Sorghum vulgare
Pers., this fungus failed to sporulate in one-species pot cultures with this
plant. The inability of arbuscular mycorrhizal fungi living in trap cultures
to sporulate in single-species pot cultures probably results from the especially
high selective nature of the latter ones, as Koske et al. (1997) and Koske
(pers. comm.) suggested. The factor most limiting establishment of mycorrhizae
in single-species pot cultures seems to be the lack of microflora of the soil
from which a given fungus originated. Soil microorganisms have been demonstrated
to hasten mycorrhizal fungus spore germination (Azcon-Aguilar et al. 1986)
and root colonization (Azcon-Aguilar and Barea 1985).
DISTRIBUTION.
In Poland, Gl. fuegianum has been found only in three soil samples
collected from the root zone of J. communis growing in the inland
dunes of the Kampinos National Park (52o18'N, 20o46'E; (Blaszkowski et al.
1998).
Glomus fuegianum
has originally been found in Argentina (Pegler et al. 1993). However, most
records of of this fungus come from the United Kingdom (Godfrey 1957; Pegler
et al. 1993) where it was considered to be rather widespread. Additionally,
Gl. fuegianum has been found in New Zealand (Hall 1977) and Australia
(McGee and Trappe 2002). However, the finding of this fungus in only three
of over 2500 soil samples collected in different regions of Europe, Africa,
Asia, and the U.S.A. (Blaszkowski, pers. observ.) indicates Gl. fuegianum
to be an extremely rarely occurring soil fungus in the world.
NOTES.
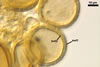
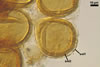
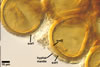
The most closely related arbuscular mycorrhizal fungi producing
spores similar in appearance and arranged in structures resembling the spore
groups of Gl. fuegianum are Gl.
rubiforme (Gerd. & Trappe) R.T. Almeida & N.C. Schenck and Gl.
sinuosum (Gerd. & B.K. Bakshi) R.T. Almeida & N.C. Schenck (Almeida and Schenck
1990; Wu 1993a, b). Although both Gl. fuegianum and Gl. rubiforme
form spores from a thick-walled, inflated hypha, the latter species lacks
the hyphal mantle. Glomus sinuosum spores are mantled by sinuous
hyphae reminiscent of those present in Gl. fuegianum, but the spores
of Gl. sinuosum originate from a mass of interwoven hyphae, not from
an inflated hypha as Gl. fuegianum.
REFERENCES
Almeida R. T., Schenck
N. C. 1990. A revision of the genus Sclerocystis (Glomaceae, Glomales).
Mycologia 82, 703-714.
Azcon-Aguilar C., Barea
J. M. 1985. Effect of soil microorganisms on formation of vesicular-arbuscular
mycorrhizas. Trans. Brit. Mycol. Soc. 84, 536-537.
Azcon-Aguilar C., Diaz-Rodriguez
R. M., Barea J. M. 1986. Effect of soil microorganisms on spore germination
and growth of the vesicular-arbuscular mycorrhizal fungus Glomus mosseae.
Trans. Brit. Mycol. Soc. 86, 337-340.
Blaszkowski J., Madej
T., Tadych M. 1998. Entrophospora baltica sp. nov. and Glomus
fuegianum, two species in the Glomales from Poland. Mycotaxon 68, 165-184.
Godfrey R. M. 1957. Studies
of British species of Endogone. I. Morphology and taxonomy. Trans.
Br. Mycol. Soc. 40, 117-135.
Hall I. R. 1977. Species
and mycorrhizal infections of New Zealand Endogonaceae. Trans. Br. Mycol.
Soc. 68, 341-356.
Koske R. E., Gemma J.
N., Jackson N. 1997. Mycorrhizal fungi associated with three species of turfgrass.
Can. J. Bot. 75, 320-332.
McGee P. A., Trappe J.
M. 2002. The Australian zygomycetous mycorrhizal fungi. II. Further Australian
sporocarpic Glomaceae. Aust. Sys. Bot. 15, 115-124.
Pegler D. N., Spooner
B. M., Young T. W. K. 1993. British truffles. A revision of British hypogeous
fungi. Royal Botanic Gardens, Kew. 216 pp. + 26 plates.
Wu C.-G. 1993a. Glomales
of Taiwan: III. A comparative study of spore ontogeny in Sclerocystis
(Glomaceae, Glomales). Mycotaxon 47, 25-39.
Wu C.-G. 1993b. Glomales
of Taiwan: IV. A monograph of Sclerocystis (Glomaceae). Mycotaxon
49, 327-349.