GERMINATION.
Not observed.
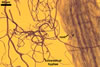
MYCORRHIZAE.
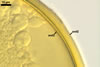
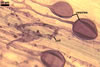
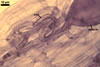
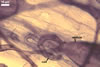
In roots of Plantago lanceolata L., the mycorrhizae
of Gl. geosporum consisted of arbuscules, vesicles, as well as intra-
and extraradical hyphae. The arbuscules and vesicles were patchily distributed
along the roots examined. The intraradical hyphae were evenly distributed
and frequently formed coils. All the structures stained moderately (arbuscules)
to intensively in 0.1% trypan blue.
DISTRIBUTION. In
Poland, Gl. geosporum has been revealed in many both cultivated and
uncultivated sites (Blaszkowski 1993).
Literature data indicate
that Glomus geosporum has a worldwide distribution. This fungus has
originally been described from spores isolated from among root of Hordeum
vulgare L. cultivated in Scotland (Nicolson and Gerdemann 1968). Glomus
geosporum has also been reported from cultivated and natural sites of,
e. g., Canada (Hamel et al. 1994; Molina et al. 1978), U. S. A. (Hetrick and
Bloom 1986;Ho 1987; Koske and Tews 1987; Miller at al. 1985; Molina et al.
1978; Schenck and Smith 1981; Walker et al. 1982), Brazil (Moreira-Souza et
al. 2003), Italy (Puppi and Riess 1982), The Netherlands and Germany (Hildebrandt
et al. 2001; Oehl et al. 2003), France and Switzerland (Oehl et al. 2003),
Israel (Blaszkowski et al. 2001; Haas and Menge (1990), China (Wu et al. 2002),
and New Zealand (Hall 1977; Hayman 1978; Johnson 1977).
NOTES.
When seen under a dissecting microscope, spores of Gl.
geosporum most resemble in size and colour those of Gl.
verruculosum Blaszk. (Blaszkowski and Tadych 1997). The two fungi also have
a 3-layered spore wall structure. However, the outermost evanescent spore
wall layer of Gl. geosporum stains reddish
white to pale red in Melzer's reagent, whereas that of Gl. verruculosum
i nonreactive in this reagent. Additionally, the inner surface of the innermost
spore wall layer of Gl. geosporum is smooth, and that of the innermost
wall layer of Gl. verruculosum spores is ornamented with warts.
REFERENCES
Blaszkowski J. 1993.
Comparative studies of the occurrence of arbuscular fungi and mycorrhizae
(Glomales) in cultivated and uncultivated soils of Poland. Acta Mycol. 28,
93-140.
Blaszkowski J., Tadych
M. 1997. Glomus multiforum and G. verruculosum, two new
species from Poland. Mycologia 89, 804-811.
Blaszkowski J., Tadych
M., Madej T., Adamska I., Iwaniuk A. 2001. Arbuscular mycorrhizal fungi (Glomales,
Zygomycota) of Israeli soils. Mat. II Polsko-Izraelskiej Konf. Nauk. nt. „Gospodarowanie
zasobami wodnymi i nawadnianie roslin uprawnych”. Przeglad naukowy Wydz.
Inz. Ksztalt. Srod. 22, 8-27.
Haas J. H., Menge J.
A. 1990. VA-mycorrhizal fungi and soil characteristics in avocado (Persea
americana Mill.) orchard soils. Plant and Soil 127, 207-212.
Hall I. R. 1977. Species
and mycorrhizal infections of New Zealand Endogonaceae. Trans. Br. Mycol.
Soc. 68, 341-356.
Hamel C., Dalpé
Y., Lapierre C., Simard R. R., Smith D. I. 1994. Composition of the vesicular-arbuscular
mycorrhizal fungi population in an old meadow as affected by pH, phosphorus
and soil disturbance. Agric. Ecosys. Environ. 49, 223-231.
Hayman D. S. 1978. Mycorrhizal
populations of sown pastures and native vegetation in Otago, New Zealand.
N. Z. J. Agric. Res. 21, 271-276.
Hetrick B. A. D., Bloom
J. 1986. The influence of host plant on production and colonization ability
of vesicular-arbuscular mycorrhizal spores. Mycologia 78, 32-36.
Hildebrandt U., Janetta
K., Ouziad F., renne B., Nawrath K., Bothe H. 2001. Arbuscular mycorrhizal
colonization of halophytes in Central European salt marshes. Mycorrhiza 10,
175-183.
Ho I. 1987. Vesicular-arbuscular
mycorrhizae of halophytic grasses in the Alvord esert of Oregon. Nortwest
Sci. 61, 148-151.
Johnson P. N. 1977. Mycorrhizal
Endogonaceae in a New Zealand forest. New Phytol. 78, 161-170.
Koske R. E., Tews L.
L. 1987. Vesicular-arbuscular mycorrhizal fungi of Wisconsin sandy soils.
Mycologia 79, 901-905.
Miller D. D., Domoto
P. A., Walker C. 1985. Mycorrhizal fungi at eighteen apple rootstock plantings
in the United States. New Phytol. 100, 379-391.
Molina R. J., Trappe
J. M., Strickler G. S. 1978. Mycorrhizal fungi associated with Festuca
in the western U.S. and Canada. Can. J. Bot. 56, 1691-1695.
Moreira-Souza M., Truem
S. F. B., Gomes-da-Costa S. M. Cardoso E. J. B. N. 2003. Arbuscular mycorrhizal
fungi associated with Araucaria angustifolia (Bert.) O. Ktze. Mycorrhiza
13, 211-215.
Nicolson T. H., Gerdemann
J. W. 1968. Mycorrhizal Endogone species. Mycologia 60, 313-325.
Oehl F., Sieverding E.,
Ineichen K., Mader P., Boller T., Wiemken A. 2003. Impact of land use intensity
on the species diversity of arbuscular mycorrhizal fungi in agroecosystems
of Central Europe. Appl. Environ. Microbiol.69, 2816-2824.
Puppi G., Riess S. 1982.
Notes on the occurrence of endogonaceous spores and vesicular-arbuscular mycorrhizal
associations in woodland sites in the middle valley of the Tiber (Italy).
Acc. Lincei-Rend. Sc. fis. mat. e nat. 22, 279-286.
Schenck N. C., Smith
G. S. 1981. Distribution and occurrence of vesicular-arbuscular mycorrhizal
fungi on Florida agricultural crops. Soil Crop Sci. Soc. Florida, Proc. 40,
171-175.
Walker C., Mize C. W.,
McNabb H. S. 1982. Populations of endogonaceous fungi at two localities in
central Iowa. Can. J. Bot. 60, 2518-2529.
Wu T., Hao W., Lin X.,
Shi Y. 2002. Screening of arbuscular mycorrhizal fungi for the revegetation
of eroded red soils in subtropical China. Platn and Soil 239, 225-235.