GERMINATION.
Unknown.
MYCORRHIZAE.
Glomus glomerulatum has formed vesicular-arbuscular mycorrhizae in a one-species culture with Pueraria phaseoloides (Roxb.) Benth as the host plant (Sieverding 1987).
PHYLOGENETIC POSITION. Unknown.
DISTRIBUTION. The holotype of Gl. glomerulatum has been designated from spores produced in the culture no. C-163-4 with the host plant P. phaseoloides grown in the Centro Internacional de Agricultura Tropical, Valle, Palmira, Colombia (Sieverding 1987). The spores used to establish this culture were extracted from pot cultures inoculated with soil from an unspecified site in the eastern plain of Colombia, Vichada, near the Meta river. Additionally, Dodd et al. (1990) identified Gl. glomerulatum associated with roots of Sorghum sp. growing in acid-infertile soils in the savanna ecosystem in the eastern plains of Colombia, and Gai et al. (2006) reported this fungus from China.
NOTES. The characterization of Gl. glomerulatum presented above was made based on the original description of this fungus (Sieverding 1987) and its specimens [vials C-163-4(1) and C-163-4(4) with sporocarps immersed in lactic acid] obtained from Dr. E. Sieverding, Institute for Plant Production and Agroecology in the Tropic and Subtropics, University of Hohenheim, Germany. Most of the spores were in a good condition. A part of them was highly perforated by soil microparasites.
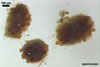
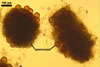
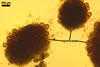
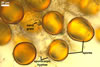
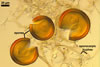
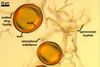
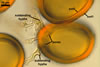
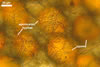
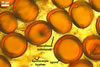
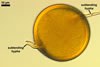
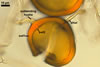
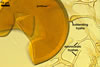
The distinctive character of Gl. glomerulatum is the formation of small and coloured spores only intercalary along its sporocarpic hyphae and, thereby, spores always possessing two subtending hyphae. The species of the genus Glomus producing coloured spores in sporocarps also are Gl. ambisporum G.S. Sm. & N.C. Schenck, Gl. aureum Oehl & Sieverd., Gl. australe (Berk.) S.M. Berch, Gl. avelingiae R.C. Sinclair, Gl. badium Oehl, Redecker & Sieverd., Gl. bagyarajii V.S. Mehrotra, Gl. formosanum C.G. Wu & Z.C. Chen, Gl. fuegianum (Speg.) Trappe & Gerd., Gl. heterosporum G.S. Sm. & N.C. Schenck, Gl. microcarpum Tul. & C. Tul., Gl. multicaule Gerd. & B.K. Bakshi, Gl. pallidum I.R. Hall, Gl. proliferum Dalpe & Declerck, Gl. rubiforme (Gerd. & Trappe) R.T. Almeida & N.C. Schenck, and Gl. spinosum H.-T. Hu . However, the sporocarps of G. autrale , G. formosanum , G. fuegianum , and G. microcarpum contain a peridium (Berch and Fortin 1986; Blaszkowski 2003; Blaszkowski et al. 1988; McGee and Trappe 2002; Wu and Chen 1986 vs. no peridium in Gl. glomerulatum ), those of Gl. australe, Gl. avelingiae, Gl. microcarpum, Gl. pallidum and Gl. spinosum are larger [1.0-1.2 x 1.8-2.2 mm in Gl. avelingiae to 1-25 mm in Gl. pallidum (Berch and Fortin 1986; Blaszkowski, pers. observ.; Hall 1977; Hu 2002; McGee and Trappe 2002; Sinclair et al. 2000; Oehl et al. 2003 vs. (290-)400 x 500(-675) µm in Gl. glomerulatum (Sieverding 1987)], and those of Gl. ambisporum, Gl. heterosporum, and Gl. fuegianum have in their centre a core consisting of either interwoven hyphae (the two former species) or a thick-walled, inflated hypha (the third fungus), from which spores develop (Blaszkowski 2003; Blaszkowski et al. 1998; Smith and Schenck 1985 vs. no such a centre in Gl. glomerulatum ). Additionally, both Gl. ambisporum and Gl. heterosporum are dimorphic fungi, producing two types of glomoid spores, a phenomenon not found in Gl. glomerulatum.
Some of the species compared here also differ in colour of their spores. Spores of Gl. ambisporum, Gl. badium, and Gl. multicaule are darker [dark brown to black, brownish orange (9C8) to reddish brown (8E8), and dark brown, respectively; Blaszkowski 2003; Gerdemann and Bakshi 1976; Oehl et al. 2005; Smith and Schenck 1985 vs. light orange (5A5) to golden yellow (5B8) in Gl glomerulatum ], and spores of Gl. proliferum are hyaline (Declerck et al. 2000) to pale yellow (Walker, pers. comm.).
Still other differences between Gl. glomerulatum and the other species listed above expose the composition and properties of the wall structure of their spores. Similarly as in Gl. glomerulatum, spores of Gl. aureum, Gl. australe, Gl. fuegianum, Gl. heterosporum, Gl. pallidum, and Gl. rubiforme have a 2-layered wall (Blaszkowski 2003; Blaszkowski et al. 1998; Blaszkowski, pers. observ.; Gerdemann and Trappe 1974; Hall 1977; McGee and Trappe 2002; Oehl et al. 2003; Smith and Schenck 1985). However, the spore wall of none of these species possesses the flexible inner layer of the spore wall of Gl. aureum. Although such a layer occurs in the wall structure of spores of Gl. ambisporum, Gl. avelingiae, Gl. badium, Gl. bagyarajii, Gl. proliferum, and Gl. spinosum, it is surrounded with either three (in Gl. bagyarajii and Gl. proliferum) or two other layers (in the other fungi; Blaszkowski 2003; Declerck et al. 2000; Mehrotra 1997; Oehl et al. 2005; Sinclair et al. 2000; Smith and Schenck 1985 vs. only one layer in Gl. glomerulatum). Spores of Gl. formosanum, Gl. microcarpum, and Gl. multicaule are descried to be 1-layered (Berch and Fortin 1984; Gerdemann and Bakshi 1976; Wu and Chen 1986).
REFERENCES
Berch S. M., Fortin J. A. 1984. A lectotype for Glomus microcarpum (Endogonaceae, Zygomycetes). Mycologia 76, 190-193.
Blaszkowski J. 2003. Arbuscular mycorrhizal fungi (Glomeromycota), Endogone , and Complexipes species deposited in the Department of Plant Pathology, University of Agriculture in Szczecin, Poland. http://www.agro.ar.szczecin.pl/~jblaszkowski.
Blaszkowski J., Madej T., Tadych M. 1998. Entrophospora baltica sp. nov. and Glomus fuegianum, two species in the Glomales from Poland. Mycotaxon 68, 165-184.
Declerck S., Cranenbrouck S., Dalpé Y., Séguin S., Grandmougin-Ferjani A., Fontaine J., Sancholle M. 2000. Glomus proliferum sp. nov.: a description based on morphological, biochemical, molecular and monoxenic cultivation data. Mycologia 92, 1178-1187.
Gerdemann J. W., Bakshi B. K. 1976. Endogonaceae of India: two new species. Trans. Brit. Mycol. Soc. 66, 340-343.
Gerdemann J. W., Trappe J. M. 1974. The Endogonaceae in the Pacific Northwest. Myc. Memoir 5, 1-76.
Hall I. R. 1977. Species and mycorrhizal infections of New Zealand Endogonaceae. Trans. Brit. Mycol. Soc. 68, 341-356.
Hu H.-T. 2002. Glomus spinosum sp. nov. in the Glomaceae from Taiwan. Mycotaxon 83, 159-164.
McGee P. A., Trappe J. M. 2002. The Australian zygomycetous mycorrhizal fungi. II. Further Australian sporocarpic Glomaceae. Austral. Sys. Bot. 15, 115-124.
Mehrotra V. S. 1997. Glomus bagyarajii sp. nov., a new species of Glomaceae (Glomales, Zygomycees) from India. Philip. J. Sci. 126, 233-242.
Oehl F., Wiemken A., Sieverding E. 2003. Glomus aureum, a new sporocarpic arbuscular mycorrhizal fungal species from European grasslands. J. App. Bot. 77, 111-115.
Oehl F., Redecker D., Sieverding E. 2005. Glomus badium, a new sporocarpic mycorrhizal fungal species from European grasslands with higher soil pH. J. Appl. Bot. and Food Quality 79, 38-43.
Sinclair R. C., Greuning Van J. V., Eicker A. 2000. A new species of sporocarpic Glomales from South Africa. Mycotaxon 74, 337-342.
Smith G. S., Schenck N. C. 1985. Two new dimorphic species in the Endogonaceae: Glomus ambisporum and Glomus heterosporum. Mycologia 77, 566-574.
Sieverding E. 1987. A VA-mycorrhizal fungus, Glomus glomerulatum sp. nov., with two hyphal attachments and spores formed only in sporocarps. Mycotaxon 29, 73-79.