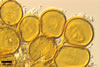
Glomus microcarpum Tul. & C. Tul.
 |
 |
 |
 |
In PVLG
|
|||
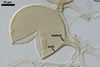
Glomus microcarpum Tul. & C. Tul.
 |
 |
 |
 |
In PVLG
|
|||
Sporocarps 180-450 x 350-730 µm, without a peridium.
Layer 1 evanescent, hyaline, (0.5-)1.0(-1.5) µm thick.
Layer 2 laminate, smooth, pale yellow (3A3) to golden yellow (5B7), (1.0-)1.9(-2.9) µm thick.
 |
 |
In PVLG |
|
Wall of subtending hypha pale yellow (3A3) to golden yellow (5B7); (2.2-)2.4(-2.9) µm thick at the spore base; composed of two layers (shwl1 and 2), continuous with spore wall layers 1 and 2.
Pore open or closed by a curved septum, continuous with the innermost sublayers of the laminate spore wall layer 2.
GERMINATION. Not observed.
MYCORRHIZAE. Unknown. No literature data exists of mycorrhizae of Gl. microcarpum.
DISTRIBUTION. In Poland, Gl. microcarpum has been found in ca. 40 sites, including both cultivated fields and those with natural vegetation (Blaszkowski 1993, 1994; Blaszkowski et al. 2002; Tadych and Blaszkowski 2000; Iwaniuk and Blaszkowski, unpubl.).
Glomus microcarpum is widely distributed in the world, although it does not occur regularly. Most reports of the presence of this fungus come from the U. S. A. (Hetrick and Bloom 1983; Menge et al. 1977; Molina et al. 1978; Nicolson and Schenck 1979; Stahl and Christensen 1982; Walker et al. 1982). It has also been found in Canada (Molina et al. 1978), Israel (Blaszkowski et al. 2001), Madras, India (Mohankumar et al. 1988), New South Wales, Australia (Hayman and Stovold 1979), and Tasmania (Gerdemann and Trappe 1974).
Apart from the the data regarding Poland (listed above), there are only a few reports of the occurrence of Gl. microcarpum in Europe. The Tulasnes (Tulasne and Tulasne 1845) originally described this fungus from spores collected in Italy and France. Then, Gl. microcarpum has many times been recorded in Europe. However, Thaxter (1922) suggested many of the collections to represent other species. Godfrey (1957) frequently found this fungus in the Bristol area (Great Britain). Puppi and Riess (1987) revealed Gl. microcarpum in dunes of Italy. Additionally, Gl. microcarpum has been found in Sweden and Norway (Eckblad 1987; Kers 1983).
NOTES. Glomus microcarpum is the type species of the genus Glomus. The most distinctive characters of Gl. microcarpum are its small, not exceeding 55 µm diam, yellow-coloured spores, usually arranged in clusters. The clusters collected by the author of this website contained from 14 to more than 100 spores. In such clusters, spores originated from variously inflated or straight and tangled hyphae, 5-10 µm wide. No sporocarps with a peridium were found. Berch and Fortin (1984) segregated a lectotype of Gl. microcarpum from the original collections by the Tulasnes, among which sporocarps enclosed by a peridium occurred. According to Berch and Fortin (1984), the peridium of Gl. microcarpum more frequently is present in larger sporocarps. Morton (1988) suggested that age of sporocarps is an important factor affecting the peridium development.
The presence of two layers in the wall structure of Gl. microcarpum spores found by the author of this website contradicts most earlier reports on the 1-layered spore wall nature of this fungus. Koske et al. (1986) stated that Gl. microcarpum forms two spore walls, with an outer unit wall sensu Walker (1983). However, in young spores, an evanescent layer highly resembles a permanent, unit layer.
REFERENCES
Berch S. M., Fortin J. A. 1984. A lectotype for Glomus microcarpum (Endogonaceae, Zygomycetes). Mycologia 76, 190-193.
Blaszkowski J. 1993. Polish Glomales 12. Glomus macrocarpum Tul. et Tul. and Glomus microcarpum Tul. et Tul. Bull. Pol. Ac. Sci. Biol. Sci. 41, 29-39.
Blaszkowski J. 1994. Arbuscular fungi and mycorrhizae (Glomales) of the Hel Peninsula, Poland. Mycorrhiza 5, 71-88.
Blaszkowski J., Adamska I., Czerniawska B. 2002. Arbuscular mycorrhizal fungi (Glomeromycota) of the Vistula Bar. Acta Mycol. 37, 39-62.
Blaszkowski J., Tadych M., Madej T., Adamska I., Iwaniuk A. 2001. Arbuscular mycorrhizal fungi (Glomales, Zygomycota) of Israeli soils. Mat. II Polsko-Izraelskiej Konf. Nauk. nt. „Gospodarowanie zasobami wodnymi i nawadnianie roslin uprawnych”. Przeglad naukowy Wydz. Inz. Ksztalt. Srod. 22, 8-27.
Eckbald F. E. 1987. Additions to Endogone and Glomus in Norway. Agarica 8, 73-75.
Gerdemann J. W., Trappe J. M. 1974. The Endogonaceae in the Pacific Northwest. Myc. Memoir 5, 1-76.
Godfrey R. M. 1957. Studies of British species of Endogone. I. Morphology and taxonomy. Trans. Br. Mycol. Soc. 40, 117-135.
Hayman D. S., Stovold C. E. 1979. Spore populations and infectivity of vesicular-arbuscular mycorrhizal fungi in New South Wales. Aust. J. Bot. 27, 227-233.
Hetrick B. A. D., Bloom J., 1983. Vesicular-arbuscular mycorrhizal fungi associated with native tall grass prairie and cultivated winter wheat. Can. J. Bot. 61, 2140-2146.
Kers L. E. 1983. Nagra svenska fynd av hypogeiska svampar. Svensk Bot. Tidskr. 77, 259-268.
Koske R. E., Gemma J. N., Olexia P. D. 1986. Glomus microaggregatum, a new species in the Endogonaceae. Mycotaxon 26, 125- 132.
Menge J., Nemec S., Davis R. M., Minassian V. 1977. Mycorrhizal fungi associated with citrus and their possible interactions with pathogens. Proc. Int. Soc. Citriculture 3, 872-876.
Mohankumar V., Ragupathy S., Nirmala C. B., Mohadevan A. 1988. Distribution of vesicular-arbuscular mycorrhizae (VAM) in the sandy beach soils of Madras coast. Cur. Sci. 57, 367-368.
Molina R. J., Trappe J. M., Strickler G. S. 1978. Mycorrhizal fungi associated with Festuca in the western U.S. and Canada. Can. J. Bot. 56, 1691-1695.
Morton J. B. 1988. Taxonomy of VA mycorrhizal fungi: classification, nomenclature, and identification. Mycotaxon 32, 267-324.
Nicolson T. H., Schenck N. C. 1979. Endogonaceous mycorrhizal endophytes in Florida. Mycologia 71, 178-198.
Puppi G., Riess S. 1987. Role and ecology of VA mycorrhizae in sand dunes. Angew. Botanik 61, 115-126.
Stahl P. D., Christensen M. 1982. Mycorrhizal fungi associated with Bouteloua and Agropyron in Wyoming sagebrush-grasslands. Mycologia 74, 877-885.
Tadych M., Blaszkowski J. 2000. Arbuscular fungi and mycorrhizae (Glomales) of the Slowinski National Park, Poland. Mycotaxon 74, 463-483.
Thaxter R. 1922. A revision of the Endogonaceae. Proc. Am. Acad. Arts Sci. 57, 291-351.
Tulasne L. R., Tulasne C. 1845. Fungi nonnulli hypogaei, novi minus cogniti act. Giorn. Bot. Ital. 2, 35-63.
Walker C. 1983. Taxonomic concepts in the Endogonaceae: spore wall characteristics in species descriptions. Mycotaxon 18, 443-455.
Walker C., Mize C. W., McNabb H. S. 1982. Populations of endogonaceous fungi at two localities in central Iowa. Can. J. Bot. 60, 2518-2529.