GERMINATION.
Not observed.
MYCORRHIZAE.
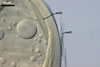
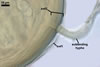
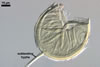
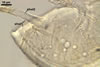
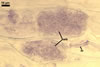
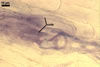
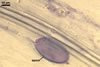
In the field, Par. laccatum has been associated with vesicular-arbuscular mycorrhizal roots of the grass species Ammophila arenaria (L.) Link, Festuca gigantea (L.) Villars, F. rubra L., Helictotrichon pubescens (Huds.) Pilg., as well as with the herbs Erodium cicutarium (L.) L'Hér., Galium aparine L., Juniperus communis L., Plantago lanceolata L., Rosa canina L., and Solidago virgaurea L. (Blaszkowski 1988, 1994; Blaszkowski et al. 2002; Tadych and Blaszkowski 2000b; Iwaniuk and Blaszkowski 2004). In one-species cultures with P. lanceolata as the host plant, mycorrhizae of Par. laccatum consisted of arbuscules, as well as intra- and extraradical hyphae. Arbuscules (arb) were not numerous and widely dispersed along the examined root fragments. They were composed of short trunks (tr) grown from parent hyphae and numerous branches with very fine tips. Intraradical hyphae (ih) extended along the main root axis. They were (2.0-)4.3(-7.6) Ám wide, straight or slightly curved, sometimes formed H- (Hb) and Y (Yb)-shaped branches and coils (c). Coils were ellipsoid, 15-22.5 x 32.5-50.0 Ám, when seen in a plane view. Extraradical hyphae were (1.5-)2.9(-5.4) Ám wide and occurred in very low abundances. Some root fragments also contained ellipsoid spores, 38-45 x 20-25 Ám, of a wall of 2.5-2.7 Ám thick. Except for extraradical hyphae and spores staining violaceous (16C5) in 0.1% trypan blue, all the other structures of the mycorrhizae remained faintly stained in this reagent. Arbuscules stained violet white (16A2) to pale violet (16A3), intraradical hyphae violet white (16A2), and coils pale violet (16A3) to light lilac (16A5).
|
|
|
|
|
|
|
|
|
In roots of P. lanceolata |
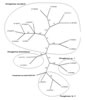
PHYLOGENETIC POSITION. SSU data unambiguously placed Glomus laccatum in the genus Paraglomus of the family Paraglomeraceae, but on a lineage well separated from Par. brasilianum and Par. occultum (Fig. 1), the only other so far known members of this genus. Consequently, Gl. laccatum is here transferred to Paraglomus and renamed Par. laccatum. These results were also confirmed by the LSU dataset and the analysis of the 5.8S gene embedded within the ITS region (data not shown). ITS data showed a heterogeneous Par. occultum represented by overall 12 sequences in the phylogenetic tree (Fig. 2). Paraglomus brasilianum is placed sister to Par. occultum. Paraglomus laccatum falls into a group of Paraglomus sequences derived from field collected roots of P. major L. and Lolium multiflorum Lam., as well as spores collected in the Thüringer Schiefergebirge (Thuringia, Germany, Fig. 2). These sequences are placed sister to two uncultured Paraglomus detected in roots of Dactylis glomerata at Lake Constance (Baden-Württemberg, Germany, Fig. 2).
DISTRIBUTION.
Paraglomus laccatum has so far rarely been found in the world and all of its literature records come from northern Poland. Blaszkowski (1988) originally described Par. laccatum from field-collected spores recovered under Festuca sp. growing in a forest at Jastrzebia Góra (55o18'N, 17o54'E). Later, Blaszkowski (1994) and Tadych and Blaszkowski (2000a) found this fungus in the field rhizosphere soils of A. arenaria and H. pubescens colonizing maritime dunes of the Hel Peninsula (54o36'-54o47'N, 18o25'-18o 48'E) and the Slowinski National Park (54o45'N, 17o26'E) adjacent to the Baltic Sea. However, trapping arbuscular fungi in pot cultures indicates that Par. laccatum is a relatively frequent inhabitant of both cultivated and uncultivated sites of northern Poland (Blaszkowski et al . 2002; Tadych and Blaszkowski 2000b; Iwaniuk and Blaszkowski 2004).
The only other record of Par. laccatum outside Poland is that of Dr. C. Walker (pers. comm.), who found this fungus in soils in Great Britain.
The infrequent disclosures of Par. laccatum in field-collected soil samples may result from the lack or irregular sporulation of this fungus under field conditions and a low persistency of its spores. In the field, a great part of AMF either do not sporulate at all or their sporulation is infrequent and seasonal (Stürmer and Bellei 1994; Stutz and Morton 1996). Paraglomus laccatum forms small, hyaline spores with a delicate spore wall that may easily be decomposed by soil microorganisms. Many soil microorganisms are parasites of AMF (Lee and Koske 1994).
NOTES. SSU, LSU and ITS data clearly separate Par. laccatum from all sequenced Glomus species and allowed to classify the original Gl. laccatum as Par. laccatum (Renker et al., in press). ITS data also suggest a close affinity of Par. laccatum to sequences of an uncultured Paraglomus species detected in the Thüringer Schiefergebirge (Börstler et al. 2006).
The most distinctive morphological character of Par. laccatum is its second structural laminate spore wall layer consisting of many easily separating sublayers. Additionally, spores of this fungus markedly glisten when mounted in water and observed under a dissecting microscope.
Spores of Par. laccatum are easy to distinguish from those of Par. brasilianum Spain & J. Miranda) J.B. Morton & D. Redecker and Par. occultum (C. Walker) J.B. Morton & D. Redecker, the only other members of the genus Paraglomus when seen under both a dissecting and a compound microscope. First, although spores of all three species are similar in size, spores of Par. laccatum remain hyaline throughout their entire life cycle and glisten, whereas those of the latter two species become slightly yellow with age and are dull (Morton 2002; Morton and Redecker 2001). Second, the outermost spore wall layer, forming the spore surface, in all the three species compared here is of the type of sloughing layers. However, this layer in Par. laccatum is least persistent and usually completely sloughed in even immature spores. Consequently, it quickly exposes the smooth and glistening upper surface of the laminate layer. In contrast, in mature spores of Par. brasilianum and Par. occultum, the outermost layer persists as a more or less deteriorated structure, thereby, making the spores dull. Third, apart from the outermost sloughing spore wall layer characterized above, the only other structure in the spore wall of Par. laccatum is a laminate layer, and the wall of spores of Par. brasilianum and Par. occultum still comprises two layers. In Par. brasilianum, the upper surface of the second spore wall layer is ornamented with minute ridges, and that of spore wall layer 2 of Par. laccatum is smooth. In Par. occultum, although spore wall layers 2 and 3 are finely laminate, none of them stratifies and thus reminds of the laminate layer of the Par. laccatum spore wall comprising many easily separating sublayers. Moreover, the sublayers of the laminate spore wall layer of Par. laccatum are much thicker than those of each of the two inner laminate spore wall layers of Par. occultum.
Apart from Par. brasilianum and Par. occultum, Par. laccatum morphologically resembles also Gl. diaphanum, Gl. minutum and Gl. viscosum, fungi producing hyaline spores of similar appearance and a more or less overlapping size range. Additionally, spores of Gl. minutum glisten, as do those of Pl. laccatum. The main difference between these fungi exists in their structural laminate spore wall layer. The sublayers of the laminate wall layer separate only in spores of Par. laccatum. Additionally, the spore wall of Gl. diaphanum and Gl. viscosum consists of three layers, and not of two as that of Par. laccatum spores. Moreover, spores of Gl. minutum and Gl. viscosum usually occur in loose aggregates, whereas Par. laccatum consistently produces only single spores. Finally, the dark staining vesicular-arbuscular mycorrhizae of Gl. minutum (Blaszkowski 2003; Blaszkowski et al. 2000) and molecular properties of spores of Gl. viscosum indicate them to be members of the order Glomerales (Schwarzott et al. 2001), while Par. laccatum is placed in Paraglomerales based on molecular analyses.
The properties of mycorrhizae of Par. laccatum also confirm the correctness of the new taxonomic status. Similarly to mycorrhizae of Par. brasilianum and Par. occultum (Morton 1985; Morton and Redecker 2001), the arbuscules and intraradical hyphae of Par. laccatum stained faintly in trypan blue, and the widely dispersed mycorrhizae of this fungus lack vesicles. The relatively thick-walled vesicle-like structures found in some mycorrhizae of Par. laccatum rather were spores of this fungus. Morton and Redecker (2001) came to similar conclusions in respect to vesicle-like structures of Par. brasilianum mycorrhizae.
REFERENCES
Blaszkowski J. 1988. Three new vesicular-arbuscular mycorrhizal fungi (Endogonaceae) from Poland. Bull. Pol. Acad. Sci. Biol. Sci. 36, 271-275.
Blaszkowski J. 1994. Arbuscular fungi and mycorrhizae (Glomales) of the Hel Peninsula, Poland. Mycorrhiza 5, 71-88.
Blaszkowski J. 2003. Arbuscular mycorrhizal fungi (Glomeromycota), Endogone and Complexipes species deposited in the Department of Plant Pathology, University of Agriculture in Szczecin, Poland. http://www.agro.ar.szczecin.pl/~jblaszkowski/.
Blaszkowski J., Adamska I., Czerniawska B. 2002. Arbuscular mycorrhizal fungi (Glomeromycota) of the Vistula Bar. Acta Mycol. 37, 39-62.
Blaszkowski J., Tadych M., Madej T. 2000. Glomus minutum, a new species in Glomales (Zygomycetes) from Poland. Mycotaxon 76, 187-195.
Börstler B., Renker C., Kahmen A., Buscot F. 2006. Species composition of arbuscular mycorrhizal fungi in two mountain meadows with differing management types and levels of plant biodiversity. Biol. Fert. Soils 42, 286-298.
Iwaniuk A., Blaszkowski J. 2004. Arbuscular fungi and mycorrhizae of agricultural soils of the Western Pomerania. Part I. Occurrence of arbuscular fungi and mycorrhizae. Acta Mycol. 39, 59-84.
Lee P. J., Koske R. E. 1994. Gigaspora gigantea: parasitism of spores by fungi and actinomycetes. Mycol. Res. 98, 458-466.
Morton J. B. 1985. Underestimation of most probable numbers of vesicular-arbuscular mycorrhizae endophytes because of non-staining mycorrhizae. Soil Biol. Biochem. 17, 383-384.
Morton J. B. 2002. International Culture Collection of Arbuscular and Vesicular-Arbuscular Mycorrhizal Fungi. Address: West Virginia University. http://invam.caf.wvu.edu.
Morton J. B., Redecker D. 2001. Two new families of Glomales, Archaeosporaceae and Paraglomaceae, with two new genera Archaeospora and Paraglomus , based on concordant molecular and morphological characters. Mycologia 93, 181-195.
Renker C, Blaszkowski J, Buscot F 2007. Paraglomus laccatum comb. nov. - a new member of Paraglomeraceae (Glomeromycota). Nova Hedwigia 84 (3-4), 395-407.
Schwarzott D. C., Walker C., Schüssler A. 2001. Glomus, the largest genus of the arbuscular mycorrhizal fungi (Glomales), is nonmonophyletic. Mol. Phylogenet. Evol. 21, 190-197.
Stutz J. C., Morton J. B. 1996. Successive pot cultures reveal high species richness of arbuscular mycorrhizal fungi in arid ecosystems. Can. J. Bot. 74, 1883-1889.
Stürmer S. L., Bellei M. M. 1994. Composition and seasonal variation of spore populations of arbuscular mycorrhizal fungi in dune soils on the island of Santa Catarina, Brazil. Can. J. Bot. 72, 359-363.
Tadych M., Blaszkowski J. 2000a. Arbuscular fungi and mycorrhizae (Glomales) of the Slowinski National Park, Poland. Mycotaxon 74, 463-482.
Tadych M., Blaszkowski J. 2000b. Arbuscular mycorrhizal fungi of the Brda river valley in the Tuchola Forests. Acta Mycol. 35, 3-23.