DITRIBUTION.
Scutellospora
calospora has originally been described from spores isolated from a cultivated
soil of Scotland (Nicolson and Gerdemann 1968). This fungus has also been
recovered from cultivated soils and those with natural vegetation located
in many other regions of the world, e. g., in USA (Gemma and Koske 1997; Gerdemann
and Trappe 1974; Nicolson and Gerdemann 1968), Canada (Dalpé 1989),
Brazil (Trufem et al. 1989), Portugal (Blaszkowski, pers. observ.), Finland
(Vestberg 1995), Spain (Blaszkowski, pers. observ.), Italy (Blaszkowski, pers.
observ.; Giovannetti 1985; Puppi and Riess 1987), Turkey, Israel (Blaszkowski,
pers. observ.), and Australia (Hall and Abbott 1984; Koske 1975).
NOTES.
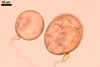
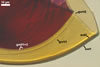
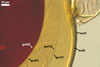
Scutellospora calospora is most similar to S. dipurpurescens
J.B. Morton & Koske. The two fungi are indistinguishable when observed
under a dissecting microscope: their spores are identical in colour, shape,
and size (Blaszkowski 1989; Franke and Morton 1994; Koske and Walker 1986;
Morton 2000; Nicolson and Schenck 1979). The spores differ only in the structure
of the germination wall 1. In S. dipurpurescens, this wall is 1-layered,
whereas the germination wall 1 of S. calospora spores includes two
layers.
REFERENCES
Blaszkowski
J. 1989. Polish Endogonaceae. I. Acaulospora bireticulata, Entrophospora
infrequens, Glomus caledonium, and Scutellispora pellucida.
Karstenia 29, 1-10.
Dalpé
Y. 1989. Inventaire et repartition de la flore endomycorhizienne de dunes
et de rivages maritimes du Quebec, du Nouveau-Brunswick et de la Nouvelle-Ecosse.
Naturaliste can. (Rev. Ecol. Syst.) 116, 219-236.
Franke
M., Morton J. B. 1994. Ontogenetic comparisons of arbuscular mycorrhizal fungi
Scutellospora heterogama and Scutellospora pellucida:
revision of taxonomic character concepts, species descriptions, and phylogenetic
hypotheses. Can. J. Bot. 72, 122-134.
Gemma
J. N., Koske R. E. 1997. Arbuscular mycorrhizae in sand dune plants of the
North Atlantic coast of the U.S.: Field and greenhouse studies. J. Environ.
Manag. 50, 251-264.
Gerdemann
J. W., Trappe J. M. 1974. The Endogonaceae in the Pacific Northwest. Myc.
Memoir 5, 1-76.
Giovannetti
M. 1985. Seasonal variations of vesicular-arbuscular mycorrhizas and Endogonaceous
spores in a maritime sand dunes. Trans. Br. Mycol. Soc. 84, 679-684.
Hall
I. R., Abbott L. K. 1984. Some Endogonaceae from south western Australia.
Trans. Br. Mycol. Soc. 83, 203-208.
Koske
R. E., Walker C. 1986. Species of Scutellospora (Endogonaceae) with
smooth-walled spores from maritime sand dunes: two new species and a redescription
of the spores of Scutellospora pellucida and Scutellospora calospora.
Mycotaxon 27, 219-235.
Morton
J. B. 2000. International Culture Collection of Arbuscular and Vesicular-Arbuscular
Mycorrhizal Fungi. West Virginia University.
Nicolson
T. H., Gerdemann J. W. 1968. Mycorrhizal Endogone species. Mycologia 60, 313-325.
Nicolson
T. H., Schenck N. C. 1979. Endogonaceous mycorrhizal endophytes in Florida.
Mycologia 71, 178-198.
Puppi G., Riess S. 1987.
Role and ecology of VA mycorrhizae in sand dunes. Angew. Botanik 61, 115-126.
Trufem S. F. B., Otomo
H. S., Malatinszy S. M. M. 1989. Fungos micorrizicos vesiculo-arbusculares
em rhizosferas de plantas em dunes do Parque Estadual da Iiha do Cardoso,
Sao Paulo, Brasil. (I) Taxonomia. Acta Bot. Bras. 3, 141-152.
Vestberg M. 1995. Occurrence
of some Glomales in Finland. Mycorrhiza 5, 329-336.