Sieverd. & S. Toro
SPORES single in the soil; formed laterally on the neck of a sporiferous saccule; light orange (5A4) to yellowish red (8B8); globose to subglobose; (112-)149(-180) µm diam.
SUBCELLULAR STRUCTURE OF SPORES consists of a spore wall and two inner germination walls.
Spore wall composed of three layers (swl1-3).
 |
 |
 |
 |
 |
 |
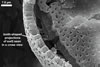
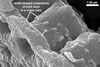
Spores seen under scanning electron microscope |
|||||
Layer 1, forming the spore surface, evanescent, hyaline, 0.6-1.6 µm thick, continuous with the wall of the sporiferous saccule neck, usually completely sloughed before the ornamentation of the laminate layer 2 is fully formed.
Layer 2 laminate, light orange (5A4) to yellowish red (8B8), 1.0-1.4 µm thick in its basic part (i. e., without the ornamentation), ornamented with tooth-shaped projections developed from stubby knobs, 2.2-2.6 µm wide and 3.0-3.2 µm high. Projections (3.9-)5.6(-7.4) µm high, (2.5-)3.2(-4.4) µm wide at their base, (3.9-)5.3(-7.1) µm wide at their top, circular, (4.0-)5.1(-6.1) µm diam, to oblong, 4.2-4.4 x 7.6-8.1 µm, with a smooth or 4-6-sided upper edge and a center cavity, 2.5-4.9 µm wide, when observed in a plane view, and 2.0-5.9 µm deep, surrounded by a lip, 1.0-2.2 µm wide, when seen in a cross view.
Layer 3 flexible, hyaline, rarely thicker than 0.8 µm, usually tightly adherent to the lower surface of the laminate layer 2, but separating from it in vigorously crushed spores.
Germination wall 1 composed of two hyaline layers (gw11 and 2).
Layer 1 flexible, 0.4-0.6 µm thick, usually tightly adherent to layer 2, which is flexible to semi-flexible and 1.2-1.4 µm thick.
Germination wall 2 comprises two hyaline layers (gw2l1 and 2).
Layer 1 flexible, 0.5-1.0 µm thick, covered with granular excrescences (a beaded layer).
Layer 2 plastic (amorphous), 3-10 µm thick in lactic acid-based mountants.
In Melzer's reagent, only layer 2 of the second germination wall stains greyish rose (11B8).
GERMINATION ORB. Not found.
SPORIFEROUS SACCULE None of the spores examined by the author of this book possessed a sporiferous saccule. According to Sieverding and Toro (1987) and Morton (2002), the sporiferous saccule of A. denticulata spores is hyaline, globose to subglobose, 80-160 µm diam, and its one-layered wall is 1.0-4.2 µm thick. The neck of the sporiferous saccule narrows towards the spore and is 14-25 µm wide at the place of its attachment, i. e., 50-100 µm from the saccule.
| In PVLG+Melzer's |
CICATRIX. It is circular to oblong, when seen in a plane view, and formed by densely packed tooth-shaped projections of the laminate spore wall layer 2 surrounding a more or less smooth, circular depression, 6.0-11.3 µm diam. At the beginning of the spore origination, only the spore wall layer 1 is continuous with the neck of the sporiferous saccule. Later, the synthesis of sublayers of the laminate layer 2 results in the formation of a lip, 2.4-3.5 µm high, between the saccule neck and the spore.
MYCORRHIZAE. According to Morton (2002) and Sieverding and Toro (1987), mycorrhizae of A. denticulata consisted of arbuscules, vesicles, as well as intra- and extraradical hyphae. Vesicles tended to localize in clusters that were patchily distributed along roots. Many were lobed or irregularly-shaped. Straight intraradical hyphae usually grew parallel to each other and were 2-4 µm diam. They connected with neighbouring hyphae via H- or Y-shaped branches. Coils mainly occurred near entry points, and their hyphae were 3-6 µm diam. In trypan blue, arbuscules stained rather lightly, and vesicles and intraradical hyphae variably.
PHYLOGENETIC POSITION. According to Oehl et al. (2006), the arbuscular mycorrhizal fungus most closely related phylogenetically to A. denticulata is A. paulinae Blaszk., which located in a sister clade.
DISTRIBUTION. The holotype of A. denticulata has been selected from the C-139-1 pot culture with a garden soil collected from the Changrila-Farm located near Tibmio, Cauca, Colombia (about 1800 m above see level) and Pueraria phaseoloides (Roxb.) Benth. as the host plant (Sieverding and Toro 1987). Other records of this fungus are those in cultivated and uncultivated soils of Canada (Klironomos et al. 2001; Marcel et al. 1988; Talukdar and Germida 1993) and China (Gai et al. 2006; Wu et al. 2002).
NOTES. The morphological and biochemical properties of spores and mycorrhizae of A. denticulata presented here were defined based on the original description of this fungus (Sieverding and Toro 1987), data formulated by Morton (2002), and examination of spores (a vial C-139-1) loaned from Dr. E. Sieverding, Institute for Plant Production and Agroecology in the Tropic and Subtropics, University of Hohenheim, Germany.
The unique structure of A. denticulata is the upper surface of the laminate spore wall layer ornamented with densely packed tooth-shaped projections directed outwards the spore.
Other known species of the genus Acaulospora producing spores of the upper surface of the structural laminate layer ornamented with projections are A. elegans, A. bireticulata, A. rehmii, A. spinosa, and A. tuberculata. However, in contrast to the tooth-shaped projections ornamenting A. denticulata spores, the ornamentation of the other species consists of crowded spines enclosed by a 4-6-sided reticulum (A. bireticulata and A. elegans ; Blaszkowski 2003; Morton 2002; Gerdemann and Trappe 1974; Rothwell and Trappe 1979), labyrinthiform folds (A. rehmii ; Blaszkowski 2003; Morton 2002; Sieverding and Toro 1987), closely packed rounded spines (A. spinosa; Morton 2002; Walker and Trappe 1981), or polygonal spines or tubercles (A. tuberculata; Janos and Trappe 1982; Morton 2002).
As results from the known phylogenetic position of A. denticulata (Oehl et al. 2006), its nearest relative is A. paulinae, a fungus also forming ornamented spores (Blaszkowski 1988, 2003). However, the ornamentation does not consist of projections, but of pits. Additionally, spores of the latter fungus are lighter-coloured [hyaline to pale yellow (3A3) vs. light orange (5A4) to yellowish red (8B8)] and much smaller [(60-)72(-95) µm diam when globose vs. (112-)149(-180)]. These fungi also differ in the occurrence in the world. While A. paulinae has many times been recorded in different regions of the world (e. g., Blaszkowski and Czerniawska 2006; Blaszkowski, unpubl. data; Koske et al. 1977; Oehl et al. 2005), only five reports of the finding of A. denticulata exist in the literature (see the section “Distribution and habitat”).
REFERENCES
Blaszkowski J. 1988. Three new vesicular-arbuscular mycorrhizal fungi (Endogonaceae) from Poland. Bull. Pol. Ac. Sci. Biol. Sci. 36, 10-12.
Blaszkowski J. 2003. Arbuscular mycorrhizal fungi (Glomeromycota), Endogone, and Complexipes species deposited in the Department of Plant Pathology, University of Agriculture in Szczecin, Poland.http://www.agro.ar.szczecin.pl/~jblaszkowski/.
Blaszkowski J., Czerniawska B. 2006. The occurrence of arbuscular mycorrhizal fungi of the phylum Glomeromycota in Israeli soils. Acta Soc. Bot. Pol. 75, 339-350.
Gai J. P., Christie P., Feng G., Li X. L. 2006. Twenty years of research on biodiversity and distribution of arbuscular mycorrhizal fungi in China: a review. Mycorrhiza 16, 229-239.
Gerdemann J. W., Trappe J. M. 1974. The Endogonaceae in the Pacific Northwest. Myc. Memoir 5, 1-76.
Janos D. P., Trappe J. M. 1982. Two new Acaulospora species from tropical America. Mycotaxon 15, 515-522.
Klironomos J. N., Hart M. M., Gurney J. E., Moutoglis P. 2001. Interspecific differences in the tolerance of arbuscular mycorrhizal fungi to freezing and drying. Can. J. Bot. 79, 1161-1166.
Koske R. E., Gemma J. N., Jackson N. 1977. Mycorrhizal fungi associated with three species of turfgrass. Can. J. Bot. 75, 320-332.
Marcel G. A., van der Heiden M. G. A., Klironomos J. N., Ursic M., Moutoglis P., Streitwolf-Engel R., Boller T., Wiemken A., Sanders I. R. 1988. Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 396, 69-72.
Morton J. B. 2002. International Culture Collection of (Vesicular) Arbuscular Mycorrhizal Fungi. West Virginia University: http://www.invam.caf.wvu.edu/.
Oehl F., Sieverding E., Ineichen K., Ris E.-A., Boller T., Wiemken A. 2005. Community structure of arbuscular mycorrhizal fungi at different soil depths in extensively and intensively managed agroecosystems. New Phytol. 165, 273-283.
Oehl F., Sýkorová Z., Redecker D., Wiemken A. 2006. Acaulospora alpina, a new arbuscular mycorrhizal fungal species characteristic for high mountainous and alpine regions of the Swiss Alps. Mycologia 98, 286-294.
Rothwell, F. M. & Trappe, J. M. 1979. Acaulospora bireticulata sp. nov. Mycotaxon 8, 471-475.
Sieverding E., Toro S. T. 1987. Acaulospora denticulata sp. nov. and Acaulospora rehmii sp. nov. (Endogonaceae) with ornamented spore walls. Angew. Bot. 61, 217-223.
Talukdar N. C., Germida J. J. 1993. Occurrence and isolation of vesicular-arbuscular mycorrhizae in cropped field soils of Saskatchewan, Canada. Can. J. Microbiol. 39, 567-575.
Walker C., Trappe J. M. 1981. Acaulospora spinosa sp. nov. with a key to the species of Acaulospora. Mycotaxon 12, 515-521.