GERMINATION.
Not
observed.
MYCORRHIZAE.
Many attempts to establish mycorrhizae of D.
spurca in
one-species pot cultures with Plantago lanceolata L. as the plant
host failed. According
to Morton (2002) and Pfeiffer et al. (1996), the mycorrhizae of D. spurca
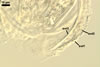
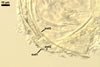
(considered as Glomus spurcum) consisted of arbuscules and intraradical hyphae staining with variable intensity
(from almost invisible to dark). No vesicles were found.
PHYLOGENETIC POSITION. According to Walker and Schüßler (2004), D. spurca is the type and so far the only species of the genus Diversispora in the family Diversisporaceae. However, results of molecular analyses indicated that other described species of arbuscular fungi grouping within the Diversispora clade are Gl. aurantium and Gl. versiforme (Blaszkowski 2003; Blaszkowski et al. 2004; Redecker et al. 2007; Schwarzott et al. 2001).
DISTRIBUTION.
In
Poland, spores of D. spurca were first found in three trap cultures
with root-rhizosphere soils of Beta vulgaris L. and Triticum
aestivum L. cultivated in Drzemin (53º15’N, 14º39’E),
Kolbacz (53o18’N, 14o49’E), and Stobno (53º26’N,
14º23’E) in the Western Pomerania district (Blaszkowski et
al. 2003; Iwaniuk and Blaszkowski 2004a, b). Subsequently, this fungus was
revealed in many trap cultures containing root-rhizosphere mixtures taken
from under Corynephorus canescens (L.) P. Beuv., Elymus arenarius
L., Eryngium maritimum L., Festuca rubra L., Phragmites
australis (Cav.) Trin. ex Steud., Rubus fructicosus L. nom.
Ambig., and Salix sp. colonizing maritime dunes adjacent to Darlówko
(54º26’N, 16º23’E) adjacent to the Baltic Sea.
Diversispora spurca has originally been discovered as Gl. spurcum in a greenhouse bed of sand
used for propagation of various ornamental plants cultivated in Arizona
(Pfeiffer et al. 1996). This fungus has also been found in maritime dunes
of Mexico (Pfeiffer et al. 1996), Hawaii (Koske and Gemma 1996), San Miguel
Island, California (Koske, pers. inform.), as well as in different other
natural ecosystems of North America, Cuba and Namibia, Africa (Kennedy et
al. 1999; Stutz and Morton 1996; Stutz et al. 2000).
NOTES.
The new combination D. spurca has been erected from Gl. spurcum mainly based on the molecular separateness of this fungus from other members of the genus Glomus (Walker and Schüßler 2004).
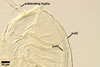
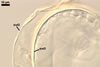
The spore wall layer 1 of D. spurca characterized here has been not included in the spore wall structure of this fungus in any earlier its description (Blaszkowski et al. 2003; Kennedy et al. 1999; Pfeiffer et al. 1996), although the presence of this structure has been mentioned. Pfeiffer et al. (1996) considered it to be a mucilaginous material formed by overlapping plate-like structures when examined with a scanning electron microscope. According to Kennedy et al. (1999), it rather is an accumulation of organic matter, because it does not stain in Melzer's reagent as a typical mucilaginous layer. The wall layer 1 of D. spurca spores examined by the author of this website occurred in almost all the specimens of this fungus coming from both Poland and those received from Dr. C. Walker, U. K.
The distinctive morphological characters of D. spurca are its hyaline to light-coloured, 3-layered spores and the second spore wall layer frequently swelling in lactic acid-based mountants.
The species of arbuscular fungi forming glomoid spores most similar to those of D. spurca in colour and size are Gl. eburneum, Gl. gibbosum, Gl. viscosum, and Paraglomus occultum. However, of them, only spores of the two later species have a 3-layered spore wall (Morton 2002; Morton and Redecker 2001; Walker 1982), as spores of D. spurca have. Moreover, none of these layers in the three fungi stains in Melzer's reagent. While the second spore wall layer of D. spurca frequently swells in lactic acid-based mountants and, thereby, separates from the laminate innermost layer of this wall, none of the spore wall layers of the two latter species swells in such mountants and separates from each other. Additionally, in the spore wall of D. spurca and Gl. viscosum, only one laminate layer occurs (Blaszkowski 2003; Kennedy et al. 1999; Morton 2002; Pfeiffer et al. 1996; Walker et al. 1995). In contrast, the spore wall of Par. occultum comprises two laminate layers (Morton and Redecker 2001).
The second spore wall layer of Gl. gibbosum also frequently swells in lactic acid-based mountants and overlies a laminate, hyaline layer of a thickness similar to that of the third laminate, hyaline innermost spore wall layer of D. spurca (Blaszkowski 1997, 2003). However, in the spore wall of the former species, a forth flexible innermost layer is still present, whose the latter fungus does not differentiate at al. Additionally, spores of D. spurca occur singly in the soil, and Gl. gibbosum produces spores singly, in loose aggregates, and in conglomerations completely enclosed by a hyphal mantle.
The spore wall of Gl. eburneum consists of only two tightly adherent layers (Kennedy et al. 1999; Morton 2002), and not of three layers, of which the second one is loosely associated with the third spore wall layer of D. spurca.
Glomus albidum, still another species that may be confused with D. spurca, also forms hyaline to pale-coloured spores (Walker and Rhodes 1981). However, they may be markedly larger [(85-)95-168(-198) x (85-)95-168(-177) µm vs. (75-)86(-110) µm diam when globose in D. spurca; Blaszkowski 2003] and their wall consists of only two layers of an equal thickness (vs. three layers different in thickness).
As mentioned in the section “Phylogenetic position”, other described members of the Glomeromycota closely related molecularly to D. spurca are Gl. aurantium and Gl. versiforme (Redecker et al. 2007; Schwarzott et al. 2001). However, the latter two species never form hyaline spores and their mature specimens are markedly darker-coloured [yellowish white (4A2) to golden yellow (5B8) and pale yellow (3A3) to deep yellow (4A8) in Gl. aurantium and Gl. versiforme, respectively; Blaszkowski 2003; Blaszkowski et al. 2004] than those of D. spurca [hyaline to pale yellow (4A3); Blaszkowski 2003]. Additionally, although the spore wall of Gl. aurantium is 3-layered and the layer overlaying the laminate one of this wall swells in lactic acid-based mountants, similarly to the second spore wall layer of D. spurca, the third and innermost component of the spore wall of the former species is a flexible, hyaline layer, which is lacking in the spore wall of the latter fungus. The third innermost spore wall component of D. spurca is a laminate layer. Thus, Gl. aurantium does not differentiate the first spore wall layer of D. spurca.
REFERENCES
Blaszkowski J. 1997.
Glomus gibbosum, a new species from Poland. Mycologia 89, 339-345.
Blaszkowski J. 2003.
Arbuscular mycorrhizal fungi (Glomeromycota), Endogone and Complexipes species deposited in the Department of Plant Pathology, University of Agriculture in Szczecin, Poland. http://www.agro.ar.szczecin.pl/~jblaszkowski/.
Blaszkowski J., Adamska
I., Czerniawska B. 2003. Glomus claroideum and G. spurcum,
arbuscular mycorrhizal fungi (Glomeromycota) new for Poland and Europe,
respectively. Acta Soc. Bot. Pol. 72, 149-156.
Blaszkowski J., Blanke V., Renker C., Buscot F. 2004. Glomus aurantium and G. xanthium, new species in Glomeromycota. 90, 447-467.
Kennedy L. J., Stutz
J. C., Morton J. B. 1999. Glomus eburneum and G. luteum,
two new species of arbuscular mycorrhizal fungi, with emendation of G.
spurcum. Mycologia 91, 1083-1093.
Koske R. E., Gemma
J. N. 1996. Arbuscular mycorrhizal fungi in Hawaiian sand dunes: Island
of Kaua’i. Pacific Sci. 50, 36-45.
Iwaniuk A., Blaszkowski J. 2004. Arbuscular fungi and mycorrhizae of agricultural soils of the Western Pomerania. Part I. Occurrence of arbuscular fungi and mycorrhizae. Acta Mycol. 39(1), 59-84.
Iwaniuk A., Blaszkowski J. 2004. Arbuscular fungi and mycorrhizae of agricultural soils of the Western Pomerania. Part II. Distribution of arbuscular fungi. Acta Mycol. 39(2), 3-18.
Morton J. B. 2002.
International Culture Collection of Arbuscular and Vesicular-Arbuscular
Mycorrhizal Fungi. West Virginia University. http://www.invam.caf.wvu.edu/.
Morton J. B., Redecker
D. 2001. Two families of Glomales, Archaeosporaceae and Paraglomaceae, with
two new genera Archaeospora and Paraglomus, based on concordant
molecular and morphological characters. Mycologia 93, 181-195.
Pfeiffer C. M., Walker
C., Bloss H. E. 1996. Glomus spurcum: a new endomycorrhizal fungus
from Arizona. Mycotaxon 59, 373-382.
Redecker D., Raab P., Oehl F., Camacho F. J., Courtecuisse R. 2007. A novel clade of sporocarp-forming species of glomeromycotan fungi in the Diversisporales lineage. Mycol. Progress 6, 35-44.
Schwarzott D., Walker C., Schüßler A. 2001. Glomus, the largest genus of the arbuscular mycorrhizal fungi (Glomales) is nonmonophyletic. Mol. Phyl. Evol. 21, 190-197.
Stutz J. C., Morton
J. B. 1996. Successive pot cultures reveal high species richness of arbuscular
mycorrhizal fungi in arid ecosystems. Can. J. Bot. 74, 1883-1889.
Stutz J. C., Copeman
R., Martin C. A., Morton J. B. 2000. Patterns of species composition and
distribution of arbuscular mycorrhizal fungi in arid regions of southwestern
North America and Namibia, Africa. Can. J. Bot. 78, 237-245.
Walker C. 1982. Species
in the Endogonaceae: a new species (Glomus occultum) and a new
combination (Glomus geosporum). Mycotaxon 15, 49-61.
Walker C., Giovannetti M., Avio L., Citernesi A. S., Nicolson T. H. 1995. A new fungal species forming arbuscular mycorrhizas: Glomus viscosum. Mycol. Res. 99, 1500-1506.
Walker C., Rhodes L.
H. 1981. Glomus albidus: a new species in the Endogonaceae. Mycotaxon
12, 509-514.
Walker C., Schüßler A. 2004. Nomenclatural clarifications and new taxa in the Glomeromycota. Mycol. Res. 108, 979-982.