GERMINATION.
Not observed.
MYCORRHIZAE.
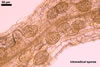
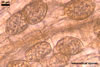
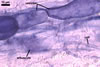
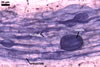
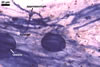
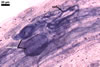
The mycorrhizae of Gl. versiforme formed in one-species
pot cultures with Plantago lanceolata L. as the plant host consisted
of arbuscules, vesicles, as well as intra- and extraradical hyphae. Arbuscules
were not numerous, patchily distributed along the roots. Vesicles were globose,
38-45 µm diam, or ellipsoid, 13-30 x 45-125 µm. Intraradical hyphae
grew parallel to each other and to the root axis, sometimes had Y-branches or
H-shaped connections, and were 2.2-9.8 µm wide. The hyphae frequently
formed coils, 20-43 x 30-110 µm. Extraradical hyphae were abundant and
1.7-3.7 µm wide. In 0.1% trypan blue, arbuscules, vesicles, coils, intra-
and extraradical hyphae stained violet white (19A2), pale violet (19A3) to violet
blue (19C8), pale violet (19A3) to deep blue (19D8), violet white (19A2) to
violet blue (19C8), and violet white (19A2) to violet blue (19C8), respectively.
PHYLOGENETIC POSITION. According to Schwarzott et al. (2001), Gl. versiforme fall into Glomus group C, also comprising Gl. spurcum C.M. Pfeiff., C. Walker & Bloss emend. L.J. Kenn., J.C. Stutz & J.B. Morton, recently renamed Diversispora spurca (C.M. Pfeiff., C. Walker & Bloss) C. Walker & Schuessler, a species of the genus Diversispora C. Walker & Schuessler within the family Diversisporaceae C. Walker & Schuessler and the order Diversisporales C. Walker & Schuessler (Walker and Schüßler 2004). Another arbuscular fungus found to be phylogenetically closely related to Gl. versiforme is Gl. eburneum L.J. Kenn., J.C. Stutz & J.B. Morton (Gamber and Leuchmann 2007). Both species are discussed below.
DISTRIBUTION. As
culturing of the field-collected root-rhizosphere soil in a trap culture indicated,
Gl. versiforme characterized here was associated in the field with
Viola tricolor L. colonizing the Baltic Sea dunes adjacent to Swinoujscie
(53o55'N, 14o14'E), although this fungus did not sporulate when the plant
species was sampled, i. e., on 13 June 1997.
The only earlier finding of Gl. versiforme in Poland is that from
the vicinity of Wroclaw (51o7’N, 17o2’E; Bucholtz 1912). However,
no detailed localization of the finding was given.
Despite the relatively not numerous literature reports of the occurrence of
Gl. versiforme, the existing data suggest that this fungus has a
worldwide distribution and is adapted to different environmental conditions,
including those of desert, subtropical, and temperate areas, as well as those
of greenhouses (Bethlenfalvay et al. 1984; Karsten 1884; Mayo et al. 1986;
Simpson and Daft 1990; Walker et al. 1982).
Glomus versiforme has originally been described from spores collected
from the surface of potting soil harboured in a greenhouse of the botanical
garden at Helsinki, Finland (Karsten 1884). Then, this fungal species has
been encountered in different states of the U.S.A. (Bethlenfalvay et al. 1984;
Daniels and Trappe 1979; Gerdemann and Trappe 1974; Mayo et al. 1986; Thaxter
1922; Walker et al. 1982), in Canada (Talukdar and Germida 1993), Mexico and
Ecuador (Daniels and Trappe 1979), Italy (Bucholtz 1912; Bonfante-Fasolo and
Vian 1984), Poland, Lithuania (Bucholtz 1912), Sweden (Kers 1985), Tasmania
(Berch and Fortin 1983), India (Simson and Daft 1990), and Australia (McGee
1986).
NOTES. When observed under a dissecting microscope, spores of Gl. versiforme are most similar to those of Gl. arenarium Blaszk. et al., Gl. caledonium (Nicol. & Gerd.) Trappe & Gerd., Gl. claroideum N.C. Schenck & G.S. Sm., Gl. eburneum, Gl. etunicatum W.N. Becker & Gerd., Gl. geosporum (Nicol. & Gerd.) C. Walker, Gl. macrocarpum Tul. & C. Tul., and Gl. verruculosum Blaszk. Spores of all these species are yellow-coloured and have a similar appearance and size range.
The main properties distinguishing Gl. versiforme from the other species listed above are the number, as well as the phenotypic and biochemical properties of components of the wall of its spores.
As in Gl. versiforme, the spore wall structure of Gl. eburneum, Gl. etunicatum and Gl. macrocarpum also is two-layered with the outer layer sloughing with age (Becker and Gerdemann 1977; Berch and Fortin 1983; Blaszkowski 1993, pers. observ; Kennedy et al. 1999; Stürmer and Morton 1997).
Mature spores of Gl. eburneum may be indistinguishable from light-coloured spores of Gl. versiforme when observed under both a dissecting and a compound microscope. Moreover, spores of these species are almost identical in size, as well as in the phenotypic and biochemical properties of the components of its wall. Additionally, mycorrhizae of these fungi stain faintly in 0.1% trypan blue (Blaszkowski, pers. observ.; Kennedy et al. 1999; Morton 2002). Thus, the only property distinguishing Gl. versiforme and Gl. eburneum is the formation of sporocarps by the latter fungus (Morton 2002).
In Gl. etunicatum, its outer spore wall layer stains intensively in Melzer's reagent and usually is present only in young spores (Blaszkowski, pers. observ.; Stürmer and Morton 1997), and that of spores of Gl. versiforme remains non-reactive in this reagent and frequently persists in mature spores (Blaszkowski et al, pers. observ.; Morton 2002). The behaviour of the sloughing outer layer of Gl. macrocarpum in Melzer's reagent is unknown. However, it swells in lactic acid-based mountants (Berch and Fortin 1983), a phenomenon not occurring in spores of Gl. versiforme. Finally, Gl. macrocarpum mainly is a hyphogeous fungus, where it most frequently produces spores in sporocarps enveloped in a peridium rather than singly in the soil (Blaszkowski 1993; Berch and Fortin 1983; Gerdemann and Trappe 1974). In contrast, spores of Gl. versiforme usually occur singly in the soil (Blaszkowski et al., pers. observ.) and only sometimes in epigeous sporocarps lacking a peridium (Bonfante-Fasolo and Vian 1984; Morton 2002). Glomus etunicatum is an ectocarpic, underground fungus (Becker and Gerdemann 1977; Stürmer and Morton 1997).
Even when observed under a compound microscope, spores of Gl. versiforme may easily be mistaken for those of Gl. arenarium, Gl. geosporum, and Gl. verruculosum. Examination of many spores of different developmental stages readily separates the four species. While Gl. versiforme produces two-layered spores, the spore wall structure of the other three species consists of three layers. The laminate layer of Gl. versiforme spores is tightly associated with a hyaline, semipermanent outer layer, and that of spores of Gl. arenarium is enveloped in two hyaline layers: an evanescent outer layer adherent to a semiflexible inner layer (Blaszkowski et al. 2001). Both the layers balloon in lactic acid-based mountants and are usually completely sloughed in mature spores.
The laminate spore wall layer of Gl. geosporum and Gl. verruculosum is covered with a single evanescent layer that is usually completely sloughed in mature spores (Blaszkowski and Tadych 1997; Blaszkowski, pers. observ.; Morton 2002; vs. a semipermanent layer usually present in mature spores of Gl. versiforme). Additionally, the laminate layer of the two former species is associated with a permanent, coloured inner layer, which is lacking in Gl. versiforme spores. In Gl. geosporum, the third spore wall layer is smooth (Blaszkowski, pers. observ.; Morton 2002; Walker 1982), whereas it is ornamented with evenly distributed warts in spores of Gl. verruculosum (Blaszkowski and Tadych 1997).
A layer adhering to the lower surface of a laminate layer also occurs in the spore wall of Gl. claroideum (Blaszkowski, pers. observ.; Morton 2002; Stürmer and Morton 1997), but is absent in the wall of Gl. versiforme spores. Additionally, the laminate layer of the spore wall of the former fungus is overlaid with two short-lived layers, and not with one relatively long-lived one as in the latter species. Finally, the outermost spore wall layer of Gl. claroideum stains intensively in Melzer's reagent, in which none of the two layers of spores of Gl. versiforme reacts.
Spores of Gl. caledonium resemble those of Gl. versiforme when the laminate, coloured structural layer of the former fungus is devoid of the hyaline outer cover consisting of three adherent layers: a mucilagenous outer layer, a rigid middle layer, and a granular inner layer (Blaszkowski, pers. observ.; Morton 1996). However, examination of many spores of different maturity readily shows this unique complex of layers. Additionally, fully developed spores of Gl. caledonium are markedly larger (180-320 µm, mean 259 µm diam; Morton 1996, 2002) than those of Gl. versiforme [(80-)106(-150) µm diam].
The only faint staining of arbuscules of Gl. versiforme mycorrhizae found in this study has also been revealed by, e. g., Hetrick et al. (1985) and Morton (2002). However, Morton (2002) did not find vesicles in roots of Zea mays L. In contrast, vesicles occurred in roots of P. lanceolata used in investigations of the authors of this website and those of nine plant species compared by Hetrick et al. (1985). In Hetrick's et al. (1985) experiments, Gl. versiforme formed vesicles only in roots of older plants, regardless of the host plant species used. In studies of the author of this website, roots of P. lanceolata with vesicles of Gl. versiforme came from at least 6-month-old cultures.
Molecularly, Gl. versiforme is most closely related to D. spurca (see the section “Phylogenetic position”). In contrast, morphologically, both species are easy to separate when the wall structure of their spores, as well as the phenotypic and biochemical properties of components of this wall are examined. Although the opinions of the number of layers covering the laminate spore wall layer of D. spurca are contradictory [one layer according to Kennedy et al. 1999 and Morton 2002 vs. two layers as Blaszkowski (2003) found], the laminate layer and the layer directly overlaying it in both fungi are very similar in both their phenotypic and biochemical properties. The main differences between these fungi reside in the persistency of the spore wall layers directly covering the laminate layer and the degree of the association of these layers with the laminate layer. Consequently, the second property highly influences the persistency of the subtending hyphae of the two fungi. Compared with Gl. versiforme, the layer directly covering the laminate spore wall layer of D. spurca is more persistent and always remains intact in even old spores (Blaszkowski 2003; Kennedy et al. 1999; vs. it usually is more or less deteriorated in mature spores of Gl. versiforme). In Gl. versiforme, the structural layer of the subtending hypha (shwl2) continuous with the laminate spore wall layer gradually thins and expands up to 8.5 µm below the spore base (Blaszkowski 2003). In contrast, in D. spurca, the laminate spore wall layer abruptly thins and stops to grow at their base, and, thereby, it does not create a sufficient support to stabilize the outer subtending hyphal wall layer continuous with the spore wall layer 2 sensu Blaszkowski (2003). Therefore, almost all crushed spores of D. spurca usually lack the subtending hypha, which detaches along with the outer wall layer of these spores.
As results from literature and the data presented above, some species compared above also differ in the phylogenetic position within the Glomeromycota. Diversispora spurca, Gl. eburneum, and Gl. versiforme belong to the Diversisporaceae (Gamber and Leuchmann 2007; Schwarzott et al. 2001; Walker and Schüßler 2004), although the latter two species were not yet formally transferred to this family. Glomus caledonium, Gl. geosporum, and Gl. verruculosum are members of Glomus group A, and Gl. claroideum comes from Glomus group B in the family Glomeraceae Piroz. & Dalpé of the order Glomerales J.B. Morton & Benny. The position of Gl. etunicatum is unclear. Of the two isolates of this fungus analyzed by Schwarzott et al. (2001), one clustered with Glomus group B, and the second one with Glomus group C. Unfortunately, the phylogenetic positions of Gl. arenarium and Gl. macrocarpum remain unknown.
REFERENCES
Becker W. N., Gerdemann
J. W. 1977. Glomus etunicatus sp. nov. Mycotaxon 6, 29-32.
Berch S. M., Fortin
J. A. 1983. Lectotypification of Glomus macrocarpum and proposal
of new combinations: Glomus australe, Glomus versiforme,
and Glomus tenebrosum (Endogonaceae). Can. J. Bot. 61, 2608-2617.
Bethlenfalvay G. J.,
Dakessian S., Pacovsky R. S. 1984. Mycorrhizae in a southern California desert:
ecological implications. Can. J. Bot. 62, 519-5124.
Blaszkowski J. 1993.
Polish Glomales XII. Glomus macrocarpum Tul. & Tul. and Glomus
microcarpum Tul. & Tul. Bull. Pol. Ac. Sci. Biol. Sci. 41, 29-39.
Blaszkowski J., Tadych
M. 1997. Glomus multiforum and G. verruculosum, two new
species from Poland. Mycologia 89, 804-811.
Blaszkowski J., Tadych
M., Madej T. 2001. Glomus arenarium, a new species in Glomales (Zygomycetes).
Acta Soc. Bot. Pol. 70, 97-101.
Bonfante-Fasolo P.,
Vian B. 1984. Wall texture in the spore of a vesicular-arbuscular mycorrhizal
fungus. Protoplasma 120, 51-60.
Bucholtz F. 1912. Beitrage
zur Kenntnis der Gattung Endogone Link. Beih. Bot. Centralbl. 29,
147-225.
Daniels
B. A., Trappe J. M. 1979. Glomus epigaeus sp. nov., a useful fungus
for vesicular-arbuscular mycorrhizal research. Can. J. Bot. 57, 539-542.
Gamber H., Leuchtmann A. 2007. Taxon-specific PCR primers to detect two inconspicuous arbuscular mycorrhizal fungi from temperate agricultural grassland. Mycorrhiza 17, 145-152.
Gerdemann J. W., Trappe
J. M. 1974. The Endogonaceae in the Pacific Northwest. Myc Memoir 5, 1-76.
Hetrick B. A. D., Bloom
J., Feyerherm S. M. 1985. Root colonization pattern of Glomus epigaeum
in nine host species. Mycologia 77, 825-828.
Karsten P. A. 1884.
Fragmenta mycologica. Hedwigia 23, 39-40.
Kennedy L. J., Stutz J. C., Morton J. B. 1999. Glomus eburneum and G. luteum, two new species of arbuscular mycorrhizal fungi, with emendation of G. spurcum. Mycologia 91, 1083-1093.
Kers L. E. 1985. Endogone
flammicorona och Glomus versiforme nya for Sverige. Svensk Bot.
Tidskr. 79, 175-185.
Mayo K., Davis R. E.,
Motta J. 1986. Stimulation of germination of spores of Glomus versiforme
by spore-associated bacteria. Mycologia 78, 426-431.
McGee P. A. 1986. Further
sporocarpic species of Glomus (Endogonaceae) from South Australia.
Trans. Brit. Mycol. Soc. 87, 123-129.
Morton J. M. 1996. Redescription
of Glomus caledonium based on correspondence of spore morphological
characters in type specimens and a living reference culture. Mycorrhiza 6,
161-166.
Morton J. B. 2002. International Culture Collection of (Vesicular) Arbuscular Mycorrhizal Fungi. West Virginia University: http://www.invam.caf.wvu.edu/.
Schwarzott D., Walker C., Schüßler A. 2001. Glomus, the largest genus of the arbuscular mycorrhizal fungi (Glomales) is nonmonophyletic. Mol. Phyl. Evol. 21, 190-197.
Simpson D., Daft M.
J. 1990. Interactions between water-stress and different mycorrhiza inocula
on plant growth and mycorrhizal development in maize and sorghum. Plant Soil
121, 179-186.
Stürmer S. L., Morton
J. B. 1997. Developmental patterns defining morphological characters in spores
of four species in Glomus. Mycologia 89, 72-81.
Talukdar N. C., Germida
J. J. 1993. Occurrence and isolation of vesicular-arbuscular mycorrhizae in
cropped field soils of Saskatchewan. Can. J. Microbiol. 39, 567-575.
Thaxter R. 1922. A revision
of the Endogonaceae. Proc. Am. Acad. Arts Sci. 57, 291-351.
Walker C. 1982. Species
in the Endogonaceae: a new species (Glomus occultum) and a new combination
(Glomus geosporum). Mycotaxon 15, 49-61.
Walker C., Mize C. W.,
McNabb H. S. 1982. Populations of endogonaceous fungi at two localities in
central Iowa. Can. J. Bot. 60, 2518-2529.
Walker C., Schüßler A. 2004. Nomenclatural clarifications and new taxa in the Glomeromycota. Mycol. Res. 108, 979-982.