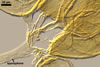
MYCORRHIZAE.
In the field, Gl. corymbiforme has been associated with vesicular-arbuscular
mycorrhizal roots of different dune plant species (Blaszkowski 1995, pers.
observ.; Blaszkowski et al. 2001, 2002a, b; Tadych and Blaszkowski 2000).
This fungus produced not numerous spores in trap cultures. However, with age
of the cultures, the sporulation disappeared. Many attempts to establish one-species
cultures of Gl. corymbiforme failed.
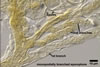
DISTRIBUTION.
In
Poland, Gl. corymbiforme has originally been found associated with
roots of plants colonizing maritime dunes adjacent to Swinoujscie (53o55'N,
14o14'E; Blaszkowski 1995). The plant species
under which Gl. corymbiforme spores occurred were Ammophila
arenaria (L.) Link, Hieracium umbellatum L., and Petasites
spurius (Retz.) Rchb. Later, this fungus has been revealed in maritime
dunes of the
Slowinski National Park (54o45’N, 17o26’E; Tadych and Blaszkowski
2000), the Vistula Bar (54o21’N, 19o14’E; Blaszkowski et al. 2002a),
and in inland dunes of the Bledowska Desert
(50o22’N, 19o34’E; Blaszkowski et al. 2002b).
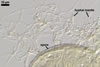
Glomus corymbiforme has also been present among spores of arbuscular
fungi isolated from dunes of the Mediterranean Sea adjacent to Karabucak-Tuzla
(36o43'N,
34o59'E), Turkey, and Tel Aviv (32º4’N, 34º46’E),
Israel (Blaszkowski et al. 2001; Blaszkowski, pers. observ.).
NOTES.
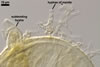
The distinctive features of Gl. corymbiforme are the hyphal mantle
enveloping individual spores and the clustered, corymbiform organization of
spores in sporocarps.
Spores of Gl. corymbiforme
are initiated from thin-walled vesicles produced terminally from straight
or branched sporophores swelling at their tips. At times, the vesicle layer
becomes thicker due to the formation of a laminated layer first and then an
innermost flexible layer. Straight, thin-walled hyphae grow from the vesicles
and the outermost layer of immature spores. Small pores connecting the spore
inside with the hyphal lumen are visible in young but mature specimens when
seen in a plan view. These hyphae branch dichotomously and become thinner
with each successive dichotomy. The hyphae of each spore intertwine with those
of neighbouring spores, forming a common mantle enveloping an aggregate. This
mantle detaches from mature spores.
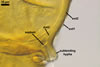
The surface of most
mature spores seen in a cross-sectional view is somewhat roughened, although
spore wall layers 1 and 2 tightly adhere to each other. These roughenesses
probably are a result of deformations of layers 1 and 2 originated during
both the development of initial mantle hyphae and the process of occlusion
of the pores connecting these hyphae with the spore contents. The latter likely
interrupts physiological first and then physical unity between the spore and
hyphae. Formation of occlusion also is a reason of detachment of hyphal sporiferous
saccule from spore in many species of the genus Acaulospora and Archaeospora
(Morton 2000).
Apart from Gl. corymbiforme,
other species of the genus Glomus forming spores enveloped by a hyphal
mantle are Gl. convolutum Gerd. & Trappe, Gl. globiferum
Koske & C. Walker, Gl. mosseae (Nicol.
& Gerd.) Gerd. & Trappe, Gl. mortonii Bent. & Hetrick,
Gl. pubescens (Sacc. & Ellis) Trappe & Gerd., Gl. sinuosa
(Gerd. & B.K. Bakshi) R.T. Almeida & N.C. Schenck, and Gl.
tortuosum N.C. Schenck & S.M. Smith. The ontogeny of the hyphal mantle
of Gl. corymbiforme is most similar to that of the mantle of Gl.
globiferum spores. Both species form a mantle from initial hyphae growing
from thin-walled vesicles or young spores (Wu and Sylvia 1993). However, in
Gl. globiferum, these hyphae bear vesiculate swelling that are absent
in Gl. corymbiforme. According to Koske and Walker (1986),
the mantle hyphae of Gl. globiferum also are darker (yellow-brown
vs. hyaline to yellowish white in G. corymbiforme) and wider [5-50
µm vs. (1.2-4.2-)1.8-4.9(-2.9-6.9) µm]. Additionally, spores of
the former species occur singly in the soil, whereas the latter fungus mainly
produces spores in sporocarps. The sinuous mantle hyphae of Gl. mortonii,
Gl. sinuosa, and Gl. tortuosum (Bentivenga and
Hetrick 1991; Gerdemann and Bakshi 1976; Schenck and Smith 1982) readily distinguish
the three species from Gl. corymbiforme with its mantle composed
of dichotomously branched hyphae. The hyphae enclosing spores of Gl. convolutum,
Gl. mosseae, and Gl. pubescens are irregularly
branched (Gerdemann and Trappe 1974) and, thereby, unlike those enveloping
Gl. corymbiforme spores. Additional features separating Gl. corymbiforme
from Gl. pubescens and Gl. sinuosa are the size
and shape of spores (Gerdemann and Bakshi 1976; Gerdemann and Trappe 1974).
Spores of Gl. corymbiforme are larger (av. 142 µm diam; range
50-220 µm diam) than those of both Gl. pubescens (20-48
x 18-45 µm) and Gl. sinuosa (45-118 x 30-83 µm). Glomus
sinuosa produces obovate to clavate spores, whereas most Gl. corymbiforme
spores are globose to subglobose.
Regardless of whether
the hyphal mantle is present, the uniquely clustered (corymbiform) organization
of Gl. corymbiforme sporocarps readily distinguishes this species
from all other described species of Glomus.
Single mature spores
of Gl. corymbiforme may be confused with those of Gl. globiferum
in which the hyphal mantle is occasionally poorly developed and difficult
to see (Koske and Walker 1986). Both species have spores similar in size,
shape, and wall structure. However, spores of the latter fungus are darker
(orange-brown to red-brown or occasionally black) than those of the former
fungus, being typically orange. Other fungal species producing spores with
three wall layers of the same types as in Gl. corymbiforme are Gl.
fasciculatum (Thaxt.) Gerd. & Trappe emend. C. Walker & Koske and Gl. pustulatum Koske et
al. (Koske et al. 1986; Walker and Koske 1987). However, spores of Gl.
fasciculatum are lighter (pale yellow to yellow-brown vs. pastel yellow
to orange), smaller [(50-)60-95(-149) x 55-90(-149) µm vs. (50-)142(-220)
µm diam], and stain in Melzer's reagent (vs. not reacting in G.
corymbiforme). Glomus pustulatum has a spore surface ornamented
with blister-like thickenings, whereas all spore wall layers in Gl. corymbiforme
are smooth.
REFERENCES
Bentivenga S. P. Hetrick
B. A. D. 1991. Glomus mortonii sp. nov., a previously undescribed
species in the Glomaceae isolated from the tallgrass prairie in Kansas. Mycotaxon
42, 9-15.
Blaszkowski J. 1995.
Glomus corymbiforme, a new species in Glomales from poland. Mycologia
87, 732-737.
Blaszkowski J., Tadych
M., Madej T., Adamska I., Iwaniuk A. 2001. Arbuscular mycorrhizal fungi (Glomales,
Zygomycota) of Israeli soils. Mat. II Polsko-Izraelskiej Konf. Nauk. nt. „Gospodarowanie
zasobami wodnymi i nawadnianie roslin uprawnych”. Przeglad naukowy Wydz.
Inz. Ksztalt. Srod. 22, 8-27.
Blaszkowski J., Adamska
I., Czerniawska B. 2002a. Arbuscular mycorrhizal fungi (Glomeromycota) of
the Vistula Bar. Acta Mycol. 37, 39-62.
Blaszkowski J., Tadych
M., Madej T. 2002b. Arbuscular mycorrhizal fungi (Glomales, Zygomycota) of
the Bledowska Desert, Poland. Acta Soc. Bot. Pol. 71, 71-85.
Gerdemann J. W., Bakshi
B. K. 1976. Endogonaceae of India: two new species. Trans. Brit. Mycol. Soc.
66, 340-343.
Gerdemann J. W., Trappe
J. M. 1974. The Endogonaceae in the Pacific Northwest. Myc. Memoir 5, 1-76.
Koske R. E., Walker
C. 1986. Glomus globiferum: a new species of Endogonaceae with a
hyphal peridium. Mycotaxon 26, 133-142.
Koske R. E., Friese
C., Walker C., Dalpé Y. 1986. Glomus pustulatum: A new species
in the Endogonaceae. Mycotaxon 26, 143-149.
Schenck N. C., Smith
G. S. 1982. Additional new and unreported species of mycorrhizal fungi (Endogonaceae)
from Florida. Mycologia 74, 77-92.
Tadych M., Blaszkowski
J. 2000. Arbuscular fungi and mycorrhizae (Glomales) of the Slowinski National
Park, Poland. Mycotaxon 74, 463-483.
Walker C., Koske R. E.
1987. Taxonomic concepts in the Endogonaceae: IV. Glomus fasciculatum
redescribed. Mycotaxon 30, 253-262.
Wu C-G., Sylvia D. M.
1993. Spore ontogeny of Glomus globiferum. Mycologia 85, 317-322.