GERMINATION
ORB. Not
found.
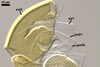
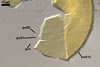
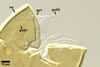
SPORIFEROUS
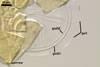
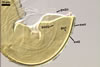
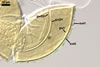
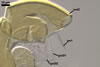
SACCULE hyaline; globose to
subglobose; 60-70 µm diam; neck 50-60 µm long, tapering from
17-20 µm diam at the saccule to 6-15 µm diam at the point of
spore attachment. Saccule usually collapses or falls off in mature spores.
CICATRIC.
A
scar, circular, 6-10 µm
diam, to oval-shaped, 6-7.5 x 7-10
µm.
MYCORRHIZAE.
Acaulospora gedanensis has been associated in the field with vesicular-arbuscular
mycorrhizal roots of many plants (Blaszkowski 1988, 1993, 1994). All attempts
to establish this fungus in both trap and one-species cultures failed.
DISTRIBUTION.
Acaulospora
gedanensis has been described from spores isolated from a rhizosphere
soil of Festuca ovina L. growing in Chalupy (54º46'N, 18º31’E)
located in the Hel Peninsula (Blaszkowski 1988). This fungus has also been
associated with many other plant species colonizing the Hel Peninsula (54º47’N,
18º25’E-54º36’N, 18º49’E) and areas adjacent to the Puck
Gulf (54º43’N, 18º24’E; Blaszkowski 1993, 1994).
There
is no information of the presence of Ac. gedanensis in other regions
of both Poland and the world.
NOTES.
Acaulospora gedanensis distinguishes its (1) small, yellow-coloured spores, (2) the rigid and fragile, one-layered inner germinal wall 1, as well as (3) the smooth outer layer and the non-reactive inner layer of the germinal wall 2.
Of the described fungi of the Glomeromycota forming coloured acaulosporioid spores, the only species producing spores similar in colour and somewhat in size to those of A. gedanensis is A. morrowiae. However, even the largest spores of the former species attain only the lower size range of those of the latter fungus [(55.0-)65.0(-75.0) µm diam when globose in A. gedanensis vs. (60.0-)75.6-80.0(-120.0) µm diam in A. morrowiae; Blaszkowski 1988; Morton 2002; Schenck et al. 1984]. The most important differences between A. gedanensis, A. morrowiae, and almost all the other known members of the genus Acaulospora reside in the phenotypic and biochemical properties of the components of the two inner germinal walls. While the first inner germinal wall of spores of A. gedanensis consists of one rigid, easily cracking layer, that of spores of the other Acaulospora spp. is generally composed of two flexible to semi-flexible layers, never cracking in even vigorously crushed specimens (Blaszkowski 2003; Morton 2002). Moreover, the upper surface of the outer layer of the second germinal wall of spores of almost all species of the genus Acaulospora is ornamented with granular excrescences (a beaded layer) and this layer adheres to either a flexible or a plastic inner layer staining more or less intensively in Melzer's reagent. In contrast, the upper surface of the outer layer of the second germinal wall of spores of A. gedanensis is smooth and this layer is associated with a flexible inner layer, but not staining in Melzer's reagent.
The rigidity and fragility of the first inner germinal wall, as well as the smooth upper surface of the outer layer of the second germinal wall and the nonreactivity of its inner layer in Melzer's reagent suggest A. gedanensis to be more closely related to members of the genus Appendicispora than to Acaulospora spp. Molecular investigations of spores of the two fungi are needed to confirm this supposition.
REFERENCES
Blaszkowski J. 1988.
Four new species of the Endogonaceae (Zygomycotina) from Poland. Karstenia
27, 37-42.
Blaszkowski J. 1993.
Comparative studies of the occurrence of arbuscular fungi and mycorrhizae
(Glomales) in cultivated and uncultivated soils of Poland. Acta Mycol. 28,
93-140.
Blaszkowski J. 1994.
Arbuscular fungi and mycorrhizae (Glomales) of the Hel Peninsula, Poland.
Mycorrhiza 5, 71-88.
Blaszkowski J. 2003. Arbuscular mycorrhizal fungi (Glomeromycota), Endogone and Complexipes species deposited in the Department of Plant Pathology, University of Agriculture in Szczecin, Poland. http://www.agro.ar.szczecin.pl/~jblaszkowski/.
Morton J. B. 2002. International Culture Collection of Arbuscular and Vesicular-Arbuscular Mycorrhizal Fungi. West Virginia University. http://www.invam.caf.wvu.edu/.
Schenck N. C., Spain J. L., Howeler R. H. 1984. Several new and unreported vesicular-arbuscular mycorrhizal fungi (Endogonaceae) from Colombia. Mycologia 76, 685-699.