DEVELOPMENT OF SPORES AND CHARACTERS OF MYCORRHIZAE
OF THE GENUS AMBISPORA
 |
 |
 |
In PVLG |
||
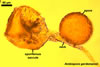
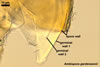
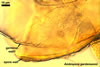
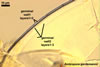
Species of the genus Ambispora are dimorphic fungi, producing both acaulosporioid and glomoid spores, i. e., spores originating similarly to those of Acaulospora and Glomus spp., respectively (Morton and Redecker 2001; Spain et al. 2006; Walker 2008). The acaulosporioid spores occur singly, and the glomoid ones singly or in loose clusters in the soil. In contrast to the sessile acaulosporioid spores of the genus Acaulospora and Archaeospora, those of Ambispora spp. develop blastically at the tip of a short branch formed at the distal end of the neck of a sporiferous saccule. This branch is called “appendix” or “pedicel”. The sporiferous saccules of Ambispora spp. originate terminally from sporogenous hyphae continuous with mycorrhizal extraradical hyphae by their swelling. Glomoid spores develop terminally from either thin-walled hyphae grown from the wall of the pedicel, branched germ tubes, or hyphae continuous with mycorrhizal extraradical hyphae (Spain et al. 2006).
 |
 |
 |
 |
 |
 |
 |
In PVLG |
In PVLG+Melzer's reagent |
In PVLG |
||||
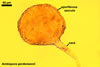
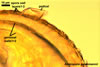
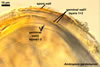
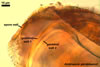
The spores of the known species of the Ambispora are globose to subglobose and coloured. Their subcellular structure consists of a 3-layered, coloured spore wall and two inner, colourless germination walls. The outermost spore wall layer, forming the spore surface, is short-lived and rarely occurs in mature spores. The other two layers also more or less deteriorate with age and, thereby, occasionally completely expose the surface of the first inner germination wall (Blaszkowski, pers. observ.; Morton 2002; Morton and Redecker 2001). This first inner germination wall is permanent and consists of two, usually tightly adherent layers. The second innermost germination wall comprises three layers, of which the middle one is finely laminate and much thicker than the outer and the lower layers. Both these layers always tightly adhere to the middle layer and are difficult to observe.
The outer spore wall completes development subsequent to the formation of the outer layer of the first inner germination wall.
The spore wall and the outer layer of the first inner germination wall of spores of Ambispora spp. are continuous with the pedicel wall layers.
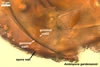
The acaulosporioid spores of Am. appendicula, the only species in which germination was recognized, germinate with a none- or branched germ tube emerging from the second inner germination wall and exiting through the pore of the pedicel. A germination structure formed between the first and the second germination walls of spores of Am. appendicula was found, but its morphological characters differ from those of the germination structures known in acaulosporioid spores of members of the Acaulospora and Ar. trappei (Spain 2003; Spain et al. 2006). The germ tubes produced frequently branch and the branches swell at their tips to form single or loose clusters of glomoid spores.
The mycorrhizae of species of the Ambispora consist of arbuscules, vesicles, as well as intra- and extraradical hyphae. All these structures stain faintly in trypan blue (Spain et al. 2006; Walker et al. 2007).
At present, the genus Ambispora includes seven species, i. e., (1) Am. appendicula, originally described as A. appendicula (Schenck et al. 1984), then erroneously synonymized with A. gerdemannii (Spain et al. 2006) to establish the acaulosporioid morph of Ar. leptoticha (Morton et al. 1997), and finally renamed Ambispora appendicula (Spain et al. 2006), (2) Am. callosa, the former Gl. callosum (Sieverding 1988) and Ap. callosa (Walker et al. 2007b), (3) Am. fecundispora, the former Gl. fecundisporum (Schenck and Smith 1982) and Ap. fecundispora (Walker et al. 2007b), (4) Am. fennica, the type species of the genus (Walker et al. 2008), (5) Am. gerdemannii, the former Gl. gerdemannii (Rose et al. 1979), later renamed Ar. gerdemannii (Morton and Redecker 2001) and Ap. gerdemannii (Spain et al. 2006), (6) Am. jimgerdemannii of its first name A. gerdemannii (Nicolson and Schenck 1979) and second one Ap. jimgerdemannii (Spain et al. 2006), and (7) Am. leptoticha originally described as Gl. leptotichum (Schenck and Smith 1982) and then renamed Ap. leptoticha (Walker et al. 2007b) .
Spain et al. (2006) concluded that the glomoid morph of Am. appendicula is not synonymous with either Gl. leptotichum or Gl. fecundisporum as Morton and Redecker (2001) considered. Consequently, the latter two species were excluded from the Ambispora and returned to the genus Glomus. However, Walker (2008) considered Gl. fecundisporum and Gl. leptotichum to be basionyms of Am. fecundispora and Am. leptoticha, respectively.
Spain et al. (2006) used six arguments to separate three of the seven species listed above (excluding Am. callosa, Am. fennica, Am. fecundispora, and Am. leptoticha) and to erect the new genus Appendicispora, later renamed Ambispora (Walker 2008). First, although Ar. trappei, the only species remained in the genus, also is a dimorphic fungus, its spores origin directly on the neck of the sporiferous saccule and, hence, are sessile, whereas those of Ambispora spp. generally develop at the tip of a pedicel. Second, in Am. appendicula and Am. gerdemannii, the wall of the pedicel is continuous with both the spore wall and the outer layer of the first inner germinal wall of their spores, a unique feature in the attachments between spores and hyphae in all mycorrhizal species of the Glomeromycota. Third, the walls of the neck and saccule of the sporiferous saccule of members of the Ambispora consist of two to three layers continuous with the wall layers of their spores, and those of Ar. trappei are continuous with only the outermost spore wall layer (Blaszkowski 2003; Morton and Redecker 2001). Forth, germination of the acaulosporioid morph of Am. appendicula, the only species of the Ambispora in which this property was recognized, is by regrowth of the germ tube through the pore of the pedicel, and not through the spore wall as in Ar. trappei (Spain 2003). Additionally, the morphological characters of the pre-germination structure of Am. appendicula differ from those of the pre-germination structure formed inside spores of Ar. trappei (Spain 2003; Spain et al. 2006). Fifth, the mycorrhizae of Ambispora spp. stain faintly in trypan blue, similarly to those of Ar. trappei (Morton 2003; Morton and Redecker 2001). However, Ambispora spp. form mycorrhizae with both arbuscules and vesicles, whereas the mycorrhizae of Ar. trappei lack vesicles at all or they form rarely (Morton and Redecker 2001). Sixth, the published results of molecular analyses of Am. jimgerdemannii (as A. gerdemannii), Am. gerdemannii (as Gl. gerdemannii), and Am. leptoticha (as Gl. leptotichum) showed them to be related with each other, but different from Ar. trappei (Redecker et al. 2000). Additionally, the differentiation of layers of the spore wall of Ambispora spp. does not proceed from its upper to lower surface as that of layers of the spore wall of Ar. trappei and other members of the Glomeromycota of known ontogenesis (Morton and Redecker 2001). In Ambispora spp., the formation of the middle spore wall layer continuous with the wall layer of the pedicel precedes the synthesis of the outermost layer forming the spore surface.
Apart from the morphological characters listed above, Ambispora spp. and Ar. trappei also differ in their range of adaptation to various environmental conditions. While literature data indicate Ambispora spp. to occur rather rarely in the world (Morton and Redecker 2001; Spain et al. 2006), Ar. trappei has a worldwide distribution and is one of the most frequently found species of the Glomeromycota in different ecosystems (Blaszkowski 2003; Blaszkowski et al. 1999; Morton and Redecker 2001).
Glomus callosum, known to form only glomoid spores, has been transferred to the genus Ambispora and named Am. callosa based on results of phylogenetic analyses of sequences of rDNA of its spores (Walker et al. 2007a).
The differentiation of acaulosporioid spores of members of the genus Ambispora generally is similar to that of spores of other members of the Glomeromycota producing acaulosporioid spores. It begins with the formation of a sporiferous saccule at the top of a sporogenous hypha continuous with a mycorrhizal extraradical hypha. Subsequently, a spore wall originates and then two inner germinal walls sequentially are formed. The unique events in the differentiation of most acaulosporioid morphs of Appendicula spp. are (1) the formation of their spores mostly at the top of a short hyphal branch grown from the sporiferous saccule neck and (2) the origination of the outermost spore wall layer after the full differentiation of its middle layer (Morton and Redecker 2001).
Spores of the glomoid morph origin identically to those of species of the genus Glomus .
REFERENCES
Blaszkowski J. 2003. Arbuscular mycorrhizal fungi (Glomeromycota), Endogone , and Complexipes species deposited in the Department of Plant Pathology, University of Agriculture in Szczecin, Poland. http://www.agro.ar.szczecin.pl/~jblaszkowski/.
Blaszkowski J., Tadych M., Madej T., Adamska I., Czerniawska B., Iwaniuk A. 1999. Acaulospora mellea and A. trappei, fungi new to the Mycota of Poland. Acta Mycol. 34, 41-50.
Morton J. B. 2002. International Culture Collection of (Vesicular) Arbuscular Mycorrhizal Fungi. West Virginia University: http://www.invam.caf.wvu.edu/.
Morton J. B., Bever J. D., Pfleger F. L. 1997. Taxonomy of Acaulospora gerdemannii and Glomus leptotichum, synanamorphs of an arbuscular mycorrhizal fungus in Glomales. Mycol. Res. 101, 625-631.
Morton J. B., Redecker D. 2001. Two families of Glomales, Archaeosporaceae and Paraglomaceae, with two new genera Archaeospora and Paraglomus, based on concordant molecular and morphological characters. Mycologia 93, 181-195.
Nicolson T. H., Schenck N. C. 1979. Endogonaceous mycorrhizal endophytes in Florida. Mycologia 71, 178-198.
Redecker D., Morton J. B., Bruns T. D. 2000. Ancestral lineages of arbuscular mycorrhizal fungi (Glomales). Mol. Phylogenet. Evol. 14, 297–301.
Rose S., Daniels B. A., Trappe J. M. 1979. Glomus gerdemannii sp. nov. Mycotaxon 8, 297-301.
Schenck N. C., Spain J. L., Howeler R. H. 1984. Several new and unreported vesicular-arbuscular mycorrhizal fungi (Endogonaceae) from Colombia. Mycologia 76, 685-699.
Spain J. L. 2003. Emendation of Archeospora and its type species, Archaeospora trappei. Mycotaxon 87, 109-112.
Spain J. L., Sieverding E., Oehl F. 2006. Appendicispora: a new genus in the arbuscular mycorrhiza-forming Glomeromycetes, with a discussion of the genus Archaeospora. Mycotaxon 97, 163-182.
Walker C. 2008. Ambispora and Ambisporaceae resurrected. Mycol. Res. 112, .....
Walker C., Vestberg M., Demircik F., Stockinger H., Saito M., Sawaki H., Nishmura I., Schüßler A. 2007. Molecular phylogeny and new taxa in the Archaeosporales (Glomeromycota): Ambispora fennica gen. sp. nov., Ambisporaceae fam. nov., and emendation of Archaeospora and Archaeosporaceae. Mycol. Res. 111, 137-13.
Walker C., Vestberg M., Schüßler A. 2007. Nomenclatural clarifications in Glomeromycota. Mycol. Res. 111, 253-255.