GERMINATION.
Not observed.
MYCORRHIZAE.
In the field, Gl. minutum has been associated with
vesicular mycorrhizal roots of Ammophila
arenaria Link, Corynephorus canescens (L.) P. B., Festuca
rubra L., F. polesica Zap., Galium ? aparine L.,
Hieracium pilosella L., H. umbellatum L., Petasites
spurius (Retz.) Rchb., and Potentilla ? anserina L.
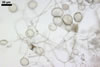
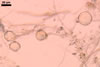
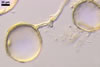
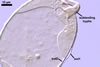
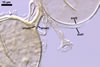
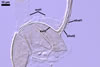
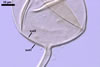
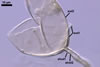
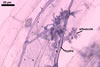
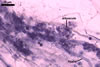
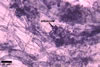
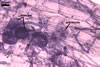
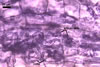
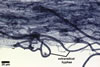
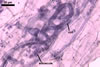
The mycorrhizae formed
in one-species cultures of this fungal species with Plantago lanceolata
L. were composed of intraradical hyphae, (3.4-)5.9(-9.1) µm wide, growing
parallel to the root axis. These hyphae sometimes formed coils, 12.5-27.5
x 17.5-55.0 µm, short branches swollen at their tip or short, perpendicular
branches connected with the neighbouring, parallel hyphae. Arbuscules were
numerous and had fine branches that were difficult to see clearly. No vesicles
were present in roots of plants even when the cultures were 6 month old. Extraradical
hyphae were 3.2-3.7 µm wide. In 0.1% trypan blue, intraradical hyphae
stained bluish white (22A2), arbuscules bluish white (22A2) to violet white
(15A2) and extraradical hyphae violet white (18A2).
DISTRIBUTION.
Glomus
minutum has sporulated abundantly in many trap cultures containing rhizosphere
soils collected from under dune plants growing near Swinoujscie (53o55’N,
14o14’E) and in the Vistula Bar (54o24'N,
19o30'E) located
in north-western and north-eastern Poland, respectively (Blaszkowski et al.
2000, 2002). However, none of the field-collected soils contained spores of
this fungus. The lack of spores of Gl. minutum in the field soils
may have resulted from three reasons. First, Gl. minutum produces
delicate, thin-walled spores that are probably quickly decomposed by soil
microorganisms. Parasitic microorganisms may significantly reduce populations
of spores of arbuscular fungi in the field (Lee and Koske 1994). Second, the
fungal species might not have been sporulating at the time of sampling. Seasonal
dependence has been observed in sporulation of arbuscular fungi (Gemma et
al. 1989). Third, Gl. minutum perhaps is a rarely or not sporulating
fungus in the field conditions. A high proportion of non-sporulating fungi
has been found in different ecosystems (Brundrett et al. 1999; Stutz and Morton
1996).
No data
exist of the presence of this fungus in other countries of the world.
NOTES.
Glomus minutum is unique due to its very small, hyaline
spores with a wall consisting of two permanent layers.
The ontogenetical development
of Gl. minutum spores expresses in relatively low increases in size
of spores and the thickness of their wall. The changes in wall thickness mainly
result from the synthesis of additional laminae in the laminate layer 2.
When observed under
a dissecting microscope, spores of Gl. minutum resemble those of D. spurca (C.M. Pfeiff., C. Walker & Bloss) C. Walker & Schuessler, Gl. diaphanum J.B. Morton & C. Walker, Gl. spurcum
C.M. Pfeiff. et al., Gl. viscosum Nicol., Paraglomus laccatum (Blaszk.) C. Renker, Blaszk. & F. Buscot, and Par.
occultum (C. Walker) J.B. Morton & D. Redecker (Blaszkowski 1988; Kennedy et
al. 1999; Morton 2002; Morton and Redecker 2001; Morton and Walker 1984; Pfeiffer
et al. 1996; Renker et al. 2007; Walker 1982; Walker et al. 1995). All the species form hyaline
to light-coloured glomoid spores and the largest spores of Gl. minutum attain
the lower level of the spore size range of the other species listed above.
However, although Gl. viscosum produces spores in loose clusters,
Gl. minutum produces them in much tighter clusters that might be
thought of as primitive spore bodies.
Examination of Gl.
minutum spores under a compound microscope readily facilitates separation
of this fungus from all the species listed above. While
the spore wall of Gl. minutum consists of two layers, that of D. spurca sensu Blaszkowski (2003), Gl.
diaphanum, Gl. viscosum, and Par. occultum
contains three. The outer layer of Gl. minutum spore wall is permanent
and semiflexible in mature spores and, thereby, resembles the outermost spore
wall layer of Gl. viscosum. However, this layer in the latter fungus
is thicker, more plastic and separates more readily from another semiflexible
layer, and not from a laminate layer as in Gl. minutum. Additionally,
the outer layer of Gl. minutum spores does not exude a mucigel-like
substance as found in Gl. viscosum. The outermost spore wall layer
of D. spurca, Gl. diaphanum, Gl. spurcum, and Par.
occultum deteriorates or sloughs with age and is usually completely or
partly absent in mature spores. Additionally, this sloughing component is
associated with either a middle permanent layer (D. spurca,
Par. occultum) or a middle laminate layer (Gl. diaphanum). Glomus
minutum also lacks the innermost semiflexible layer of Gl. diaphanum
and the innermost permanent layer of Par. occultum. Finally, the
colour of mature spores of D. spurca ranges from hyaline to pale
yellow, whereas all Gl. minutum spores remain colourless through
their entire life cycle.
The features distinguishing
Gl. minutum and Par. laccatum are properties of their spore
wall and subtending hypha. The outermost spore wall layer of the latter species
sloughs with age and usually is absent in mature specimens, and its laminate
layer is composed of easily separating and thick (ca. 0.5-2.2 µm) laminae
(vs. inseparable and very thin in Gl. minutum). The subtending hypha
of Par. laccatum compared with that of Gl. minutum is much
wider (7.4-12.9 µm vs. 4.2-8.1 µm), and its wall is less compact,
because it is formed from loose laminae continuous with those of the laminate
spore wall layer.
The mycorrhizal colonization
of Gl. minutum resembles those of D. spurca, Gl.
viscosum, and Par. laccatum in consisting of only hyphae and arbuscules with no vesicles
(Morton 2002; Pfeiffer et al. 1996; Renker et al. 2007), which are present in mycorrhizal structures
of Gl. diaphanum and Par. occultum (Morton 2002; Morton and
Redecker 2001; Morton and Walker 1984; Walker 1982). However, the mycorrhizae
of Gl. minutum stain very lightly in trypan blue, whereas those of
D. spurca and Gl. viscosum may be intensively stained.
Apart from morphological characters, the fungi compared above also differ in the phylogenetic position within the Glomeromycota. Diversispora spurca belongs to the family Diversisporaceae C. Walker & Schuessler in the order Diversisporales C. Walker & Schuessler, Gl. diaphanum and Gl. viscosum are members of Glomus groups A and B, respectively, in the family Glomeraceae Piroz. & Dalpé of the order Glomerales J.B. Morton & Benny, and Par. laccatum and Par. occultum represent the family Paraglomaceae J.B. Morton & D. Redecker in the order Paraglomerales C. Walker & Schuessler (Blaszkowski et al. 2006; Schwarzott et al. 2001; Schüßler et al. 2001; Walker and Schüßler 2004). Unfortunately, molecular properties of spores of Gl. minutum have not so far been determined.
REFERENCES
Blaszkowski J. 1988.
Three new vesicular-arbuscular mycorrhizal fungi (Endogonaceae) from Poland.
Bull. Pol. Ac. Sci. Biol. Sci. 36, 10-12.
Blaszkowski J., Renker C., Buscot F. 2006. Glomus drummondii and G. walkeri, two new species of arbuscular mycorrhizal fungi (Glomeromycota). Mycol. Res. 110, 555-566.
Blaszkowski J., Tadych
M., Madej M. 2000. Glomus minutum, a new species in Glomales (Zygomycetes)
from Poland. Mycotaxon 76, 187-195.
Blaszkowski J., Adamska
I., Czerniawska B. 2002. Arbuscular mycorrhizal fungi (Glomeromycota) of the
Vistula Bar. Acta Mycol. 37, 39-62.
Brundrett M. C., Abbott
L. K. , Jasper D. A. 1999. Glomalean mycorrhizal fungi from tropical Australia.
I. Comparison of the effectiveness and specificity of different isolation
procedures. Mycorrhiza 8, 305-314.
Gemma J. N., Koske R.
E., Carreiro M. 1989. Seasonal dynamics of selected species of VA mycorrhizal
fungi in a sand dune. Mycol. Res. 92, 317-321.
Kennedy L. J., Stutz
J. C., Morton J. B. 1999. Glomus eburneum and G. luteum,
two new species of arbuscular mycorrhizal fungi, with emendation of G.
spurcum. Mycologia 91, 1083-1093.
Lee P. J., Koske R. E.
1994. Gigaspora gigantea: parasitism of spores by fungi and actinomycetes.
Mycol. Res. 98, 458-466.
Morton J. B. 2002. International
Culture Collection of Arbuscular & Vesicular-Arbuscular Mycorrhizal Fungi.
West Virginia University.
Morton J. B., Redecker
D. 2001. Two families of Glomales, Archaeosporaceae and Paraglomaceae, with
two new genera Archaeospora and Paraglomus, based on concordant
molecular and morphological characters. Mycologia 93, 181-195.
Morton J. B., Walker
C. 1984. Glomus diaphanum: a new species in the Endogonaceae common
to West Virginia. Mycotaxon 21, 431-440.
Pfeiffer C. M., C. Walker
C., Bloss H. E. 1996. Glomus spurcum: a new endomycorrhizal fungus
from Arizona. Mycotaxon 59, 373-382.
Renker C., Blaszkowski J., Buscot F. 2007. Paraglomus laccatum comb. nov. – a new member of Paraglomeraceae (Glomeromycota). Nova Hedwigia 84 (3-4), 395-407.
Stutz J. C., Morton J.
B. 1996. Successive pot cultures reveal high species richness of arbuscular
mycorrhizal fungi in arid ecosystems. Can. J. Bot. 74, 1883-1889.
Walker C. 1982. Species
in the Endogonaceae: a new species (Glomus occultum) and a new combination
(Glomus geosporum). Mycotaxon 15, 49-61.
Walker C., Giovannetti
M., Avio L., Citernesi A. S., Nicolson T. H. 1995. A new fungal species forming
mycorrhizas: Glomus viscosum. Mycol. Res. 99, 1500-1506.