GERMINATION.
Not observed.
MYCORRHIZAE.
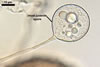
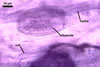
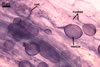
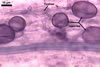
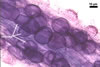
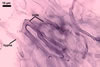
The mycorrhizae produced by Gl. trimurales in one-species
pot cultures with Plantago lanceolata L. as the plant host consisted
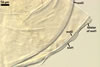
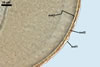
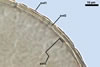
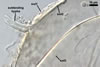
of arbuscules, vesicles, as well as intra- and extraradical hyphae. Arbuscules
were numerous, evenly distributed along the roots and stained violet-white
(17A2) to bluish- violet (18A7) in 0.1% trypan blue. Vesicles were ellipsoid,
25-85 x 30-105 µm and stained pastel-violet (19A4) to greyish-violet
(19C4) in trypan blue. Intraradical hyphae grew parallel to each other and
to the root axis, were 2.7-9.3 µm and stained violet white (18A2). The
hyphae frequently formed coils, especially at entry points. The coils were
12.0-13.7 x 25.6-31.1 µm and stained pastel-violet (18A4; when separated
from the entry points) to deep violet (18E8; at the entry points).
DISTRIBUTION.
In Poland, spores of Gl.
trimurales have been recovered from three trap cultures established using
soil samples collected from under Corynephorus canescens (L.) P.
B. (2 cultures) and Helicotrichon umbellatum (Hads.) Pilg. (1 culture)
colonizing maritime sand dunes adjacent to Swinoujscie (53o55’N, 14o14’E)
in north-western Poland (Blaszkowski et al. 2003). However, in the dunes,
Gl. trimurales sporulated only when associated with roots of H.
umbellatum (at an abundance of two spores in 100 g dry soil), as examination
of spores isolated from field-collected rhizosphere soils of the two plant
species indicated.
Glomus trimurales
probably is an extremely rarely occurring species in the world. In Poland,
it was revealed in only three of the about 2500 soil samples examined to date.
This fungal species was not found in any of the almost 900 pot trap cultures
with rhizosphere soils of maritime dune plants growing in 21 countries of
the world, including those of Africa, Asia, Europe, and U.S.A. (Blaszkowski,
pers. observ.). According to Koske (1987), Koske and Halvorson (1989) and
Koske (pers. comm.), Gl. trimurales occurred rarely in sand dunes
of New Jersey, Maryland and Virginia, but was the third most frequently recorded
species in dunes of San Miguel Island.
NOTES.
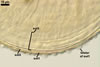
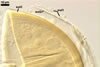
The most distinctive property of Gl. trimurales is its innermost
hyaline, stratifying laminate layer 3. The yellowish-white to golden-yellow
spore colour of this fungus comes mainly from the permanent spore wall layer
2. The lack of parts of the outermost layer 1 on the surface of some mature
spores indicates this layer to be relatively long-lived, although it is not
likely so permanent as an outermost layer of some other Glomus spp.,
e. g., Gl. pustulatum Koske
et al. (Koske et al. 1986; Blaszkowski, pers. observ.).
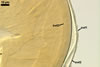
The wall of subtending
hypha of almost all the spores examined consists of only one layer continuous
with the laminate spore wall layer 3. If present, the outermost layer and
the middle layer of the subtending hyphal wall continuous with spore wall
layers 1 and 2, respectively, are visible only very closely at the spore base.
The morphological properties
of Gl. trimurales spores recovered from Poland generally correspond
to those presented in the original description of this fungus (Koske and Halvorson
1989). The exceptions are only the properties of their outermost spore wall
layer. Koske and Halvorson’s (1989) description suggests that the outermost
wall layer of Gl. trimurales spores is a pale yellow to yellow brown,
smooth, persistent, laminate layer. In contrast, in spores coming from Poland,
this layer is much lighter coloured (colourless to orange white), ornamented
with thickenings or blister outgrowths, more or less deteriorated in older
spores; and lacks any sublayers (laminae). Colour of wall layers of spores
of arbuscular fungi varies with their age and usually is darker in spores
produced in the field (Bentivenga and Morton 1995; Morton 1995). The original
protocol of Gl. trimurales (Koske and Halvorson 1989) suggests it
to be made from field-collected spores.
In the Polish specimens,
the blisters are best visible when intact, i. e., usually only in maturing
or mature spores. With age, they gradually diminish or completely disappear
because of their degeneration and sloughing. The blisters were infrequently
visible on spores isolated from the field. Examination of Gl. trimurales
spores received from Professor R. E. Koske revealed them to have a roughened
surface, and the ruggedness present are probably remnants of deteriorated
blisters. No sublayers in the outermost layer 1 of these spores were found.
Finally, the laminae of the laminate innermost layer of some of the U. S.
specimens of this fungus also are highly stratified, as in spores of this
species coming from Poland. This distinctive property has not been given by
Koske and Halvorson (1989). Of the known Glomus spp., probably only
Gl. laccatum Blaszk. produces
spores with so easily stratifying sublayers of their laminate wall layer (Blaszkowski
1988).
The darkly staining
vesicular-arbuscular mycorrhizae of Gl. trimurales for the first
time presented in this paper supports the membership of this fungus to the
genus Glomus (Morton and Redecker 2001).
When seen under a stereoscope
microscope, spores of Gl. trimurales most resemble those of Gl.
pustulatum and Gl. versiforme
(P. Karsten) S.M. Berch. The three fungi produce spores similar in colour and size,
and the ornamentation of the surface of Gl. trimurales spores closely
resembles that of Gl. pustulatum (Berch and Fortin 1983; Daniels
and Trappe 1979; Koske et al. 1986).
Examination of spore
wall structure readily separates the three fungi. The spore subcellular structure
most markedly separating the fungi is their laminate layer. In Gl. trimurales,
it is hyaline and easily stratifies due to its exceptionally loose sublayers.
In contrast, the laminate layer of Gl. pustulatum and Gl. versiforme
consists of tightly adherent, coloured laminae. Additionally, while the laminate
layer in Gl. trimurales spores is the innermost layer in
their 3-layered wall structure, that of Gl. versiforme is the second
one in its 2-layered spores. The innermost layer of spores of Gl. pustulatum
is a thin, flexible layer. Although the thickenings and outgrowths of the
outermost layer of spores of Gl. trimurales and Gl. pustulatum
are similar in appearance, size and distribution, they are formed by a hyaline
to orange white layer in the former fungus and by a yellow brown to orange
brown layer in the latter species. Additionally, none of the species considered
in this comparison possesses the coloured, permanent layer 2 of Gl. trimurales
spores.
REFERENCES
Bentivenga S. P., Morton
J. B. 1995. A monograph of the genus Gigaspora, incorporating developmental
patterns of morphological characters. Mycologia 87, 719-731.
Berch S. M., Fortin
J. A. 1983. Leptotypification of Glomus macrocarpum and proposal
of new combinations: Glomus australe, Glomus versiforme,
and Glomus tenebrosum (Endogonaceae). Canad. J. Bot. 61, 2608-2617.
Blaszkowski J. 1988.
Four new species of the Endogonaceae (Zygomycotina) from Poland. Karstenia
27, 37-42.
Blaszkowski J., Adamska
I., Czerniawska B. 2003. Glomus trimurales, an arbuscular mycorrhizal
fungus (Glomerales) new for Poland and Europe. Mycotaxon 87, 425-436.
Daniels B. A., Trappe
J. M. 1979. Glomus epigaeus sp. nov., a useful fungus for vesicular-arbuscular
mycorrhizal research. Canad. J. Bot. 57, 539-542.
Koske R. E. 1987. Distribution
of VA mycorrhizal fungi along a latitudinal temperature gradient. Mycologia
79, 55-68.
Koske R. E., Halvorson
W. L. 1989. Scutellospora arenicola and Glomus trimurales:
two new species in the Endogonaceae. Mycologia 81, 927-933.
Koske R. E., Friese
C., Walker C., Dalpé Y. 1986. Glomus pustulatum: A new species
in the Endogonaceae. Mycotaxon 26, 143-149.
Morton J. M. 1995. Taxonomic
and phylogenetic divergence among five Scutellospora species based
on comparative developmental sequences. Mycologia 87, 127-137.
Morton J. B., Redecker
D. 2001. Two families of Glomales, Archaeosporaceae and Paraglomaceae, with
two new genera Archaeospora and Paraglomus, based on concordant
molecular and morphological characters. Mycologia 93, 181-195.