
(Peck) U. Braun et S. Takamatsu
 |
MYCELIUM amphigenous, more abundant on the upper surface of laves; white, powdery, composed of loosely interwoven hyphae (hp) when young, becoming gray and compact, with many cloddy structures formedby closely interwoven hyphae when old.
 |
 |
HAUSTORIA (h) globose, elliptic, pear-shaped to irregular; hyaline to yellowish white (1A1); 5.0-10.0 x 7.5-12.0 µm.
 |
HYPHAE (hp) hyaline; (3.4-)5.7(-7.5) µm wide; branched; with many lobed and lobed-opposite appressoria (a), 4.9-5.5 x 7.2-8.1 µm.
 |
 |
 |
FOOT CELLS (fc) cylindrical, straight, sporadically slightly slanted; (7.3-)11.3 x 14.1(-18.9) µm; one to two next cells cylindrical; (8.3-)9.3 x 12.7(-16.4) µm.
 |
 |
 |
CONIDIA (c) cylindrical to ellipsoid; hyaline; (12.5-)13.1 x 35.0(-45.0) µm, developing singly.
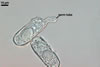 |
 |
 |
|
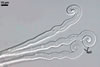
Germinated conidia
|
||
GERMINATION OF CONIDIA of the Polygoni type (Braun 1987).
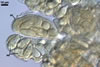
CLEISTOTHECIA scattered to gregarious, present on both surfaces of leaves; globose; (120-)130-160(-170) µm diam; burn Sienna (7D8) to English red (8D8); with a peridium composed of two layers (layers 1 and 2).
 |
 |
 |
 |
 |
Layer 1 (cl1) solid, hyaline to orange white (4A2) with branched appendages of types I and II.
Layer 2 (cl2) burn Sienna (7D8) to English red (8D8), consisting of polygonal cells, (7.8-)13.5 x 21.6(-27.6) µm when seen in a plan view.
 |
 |
 |
APPENDAGES (ap) are of two types (type 1 and 2).
Appendages of type 1 (ap1) aseptate, rigid, 23-40 per cleistothecium, distributed along its equator; straight and warted from the base to ca. the middle of their length, then slightly curved, with a smooth, wavy surface and a helically rolled apex (ap); hyaline, with a hyaline to orange white (4A2) base; (5.6-)6.7(-8.3) µm wide at the base, gradually widening to (6.1-)8.0(-10.6) µm at the apex; with a wall (1.5-)1.8(-2.2) µm thick at the base and (0.5-)0.7(-1.0) µm thick at the apex.
Appendages of type 2 (ap2) aseptate, rigid, scattered on the surface of cleistothecia; straight or slightly slanted; smooth or warted; hyaline; 21.8-52.2 µm long, 4.4-5.1 µm wide at the base, 2.4-2.9 µm wide at the apex; apex rounded or sharpened.
 |
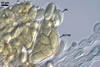 |
ASCI (ac) (5-)6-10(-19) per cleistothecium; sessile or short stalked; bitunicate; hyaline; (27.5-) x 39.2-63.8(-67.5) µm; 8-9-spored.
ASCOSPORES (as) one-celled; ellipsoid to ellipsoid-ovoid; hyaline; (7.5-)9.5-20.0(-23.2) µm.
DISTRIBUTION AND HABITAT. Erysiphe flexuosa has so far been found in two localities of northern Poland, i. e., in Szczecin ( 53º26'N, 14º35'E) and Kluki (N 54°41'N, 17°21'E), the latter placed in the Slowinski National Park (Zimmermannová-Pastircáková et al. 2002), as well as in Tarnow (50°34'N, 16°43'E) located in southern Poland (Piatek 2002). This powdery mildew fungus occasionally colonized leaves of both very young and old trees of only Aesculus hippocastanum L. Examination of other members of the Hippocastanaceae growing in Szczecin did not reveal any affection by this pathogen.
Erysiphe flexuosa commonly parasitized different species of the genus Aesculus growing in the United States of America and Canada (Braun 1987; Farr et al. 1989) and has recently been found in Germany and Switzerland (Ale-Agha et al. 2000). Bunkian (1991) recorded E. flexuosa from the Far East of Russia.
NOTES. Based on molecular examinations, Braun and Takamatsu (2000) introduced a new generic taxonomy (classification) of powdery mildew fungi. Species of all genera with pseudoidium anomorphs, i. e., Erysiphe s. str., Microsphaera, Uncinula, and Uncinuliella, were united in Erysiphe emend.
The morphological properties of the conidial and ascogenous forms of E. flexuosa found in Poland generally are within the ranges of those given by Braun (1987) and Ale-Agha et al. (2000). The exceptions are the number of asci in some cleistothecia and the wall structure of cleistothecia. Additionally, Braun’s (1987) and Ale-Agha’s et al. (2000) descriptions of E. flexuosa lack any information on conidial germination.
According to Braun (1987) and Ale-Agha et al. (2000), the cleistothecia of E. flexuosa contain 5-12 asci, whereas those found in Poland occasionally contained up to 19. The number of asci per cleistothecium in species of the order Erysiphales vary strongly and is highly correlated with the volume of the cleistothecial interior (Braun 1987).
Braun (1987) informed that the wall of cleistothecia of members of the order Erysiphales is one- or two-layered. When two-layered, the outer one is composed of dark-coloured and thick-walled polygonal cells. Moreover, Braun’s (1987) and Ale-Agha’s et al. (2000) descriptions of E. flexuosa suggest that the appendages of both types 1 and 2 of its cleistothecia develop from a dark-coloured layer consisting of polygonal cells. However, the investigations of the authors of this website clearly revealed that the dark-coloured layer comprising polygonal cells in cleistothecia of E. flexuosa is an inner layer, and their appendages grow from a colourless or light-coloured, solid outer layer. Similar two-layered wall structures with a colourless or light-coloured, solid outer layer also have cleistothecia of E. necator (Schwein) Burrill and Sawadea bicornis (Wallr.: Fr.) Homma (Gadoury and Pearson 1990, Adamska, Blaszkowski and Madej, pers. observ.). Ontogenetic investigations of Gadoury and Pearson (1990) showed that the solid outer layer of cleistothecia of E. necator originates due to gradual sealing up and, thereby, decaying of two to three outer cell layers with age. However, compared with the outer layer of E. flexuosa cleistothecia, that of cleistothecia of S. bicornis and E. necator is much thinner (Adamska, Blaszkowski and Madej, pers. observ.). In mature specimens of E. necator, this layer resembles in colour their inner layer, and hence it usually is difficult to see. Additionally, the inner layer of E. flexuosa and E. necator is compact, whereas it readily separates usually into three sublayers in S. bicornis. Further investigations of the wall structure of cleistothecia of members of the order Erysiphales are needed to utilize its differences in classification and establishment of phylogenetic relationships among species.
The pattern of germination of conidia of E. flexuosa found by the authors of this website study corresponds to the Polygoni type, which is characteristic for the whole genus of Erysiphe emend (Braun and Takamatsu 2000).
REFERENCES
Ale-Agha N., Braun U., Feige B., Jage H. 2000. A new powdery mildew disease on Aesculus spp. introduced in Europe. Cryptogamie Mycol. 21, 89-92.
Braun U. 1987. A monograph of the Erysiphales (powdery mildews). Beih. Nova Hedwigia 89, 1-700.
Braun U., Takamatsu U. 2000. Phylogeny of Erysiphe, Microsphaera, Uncinula (Erysiphae) and Cystotheca, Podosphaera, Sphaerotheca (Cystotheceae) inferred from rDNA ITS sequences – some taxonomic consequences. Schlechtendalia 4, 1-33.
Bunkian I. A. 1991. Erysiphales. In: Nizshie rasteniya, griby i mohoobraznye Sovetskogo Dal’nego Vostoka, Griby. Tom 2, Askomicety, Erizifal’nye, Klavicipital’nye, Gelocial’nye, 11-142. Nauka, Leningrad.
Farr D. F., Bills G. F., Chamuris G. P., Rossman A. Y. 1989. Fungi on plants and plant products in the United States. APS Press. American Phytopathological Society St. Paul, Minnesota USA.
Gadoury D. M., Pearson R. C. 1990. Ascocarp dehiscience and ascospore discharge in Uncinula necator. Phytopathology 80, 393-401.
Piatek M. 2002. Erysiphe flexuosa, a new for Poland powdery mildew causing disease of Aesculus hippocastanum. Polish Phytopath. Soc. 24, 67-71.
Zimmermannová-Pastircáková, Adamska I., Blaszkowski J., Bolay A., Braun U. 2002. Epidemic spread of Erysiphe flexuosa (North American powdery mildew of horse-chestnut) in Europe. Schlechtendalia 8, 39-45.