

Spain, Sieverd. & N.C. Schenck
 |
 |
 |
In PVLG |
In PVLG+Melzer's reagent |
|
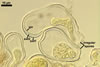
SPORES occur singly and in loose aggregates, mainly in the soil, frequently also within soil crevices, inside roots, empty spores of other species of the Glomeromycota, insect carapaces, and old seed testa. Aggregates of irregular shape, 725(-1050) x 525(-906) µm. Spores formed blastically either at the tip of a short pedicel branched from the neck of a sporiferous saccule or directly from the neck of the sporiferous saccule, when sessile. Spores hyaline; mainly globose to subglobose; (22.0-)32-90 µm diam; frequently cylindrical, ovoid, pyriform or irregular; (23.0-)28.0-95.0(-114.0) x (21.0-)28.0-80.0(-96.0) µm.
SUBCELLULAR STRUCTURE OF SPORES consists of a spore wall and one inner germinal wall.
In PVLG+Melzer's reagent |
||||||||
Spore wall composed of two hyaline layers (swl1 and 2).
Layer 1, forming the spore surface, semi-flexible, uniform, hyaline, 0.75-2.0 µm thick.
Layer 2 flexible, ca. 0.5 µm thick.
Germinal wall consists of two hyaline layers (gw1l1 and 2).
Layer 1 semi-flexible, 0.3-1.5 µm thick.
Layer 2 flexible, very thin, <0.3 µm thick, rarely separating from layer 1 and, hence, exceptionally difficult to see.
In Melzer's reagent, neither the layers of the spore wall nor any of the two layers of the inner germinal wall stain.
PEDICEL 2.8-12 µm long and 2.7-8.0 µm wide, consisting of a hyaline wall continuous with the wall of the sporiferous saccule neck.
GERMINATION ORB. Not found.
In PVLG+Melzer's reagent |
|
CICATRIX. A slightly raised collar surrounding a hole, 2.8-3.5 µm diam.
MYCORRHIZAE. Acaulospora myriocarpa formed mycorrhizae with Allium porrum L., Brachyaria sp., Coffea arabica L., Manihot esculenta Crantz, Pueraria phaseoloides (Roxb.) Benth., and Stylosanthes sp. (Schenck et al. 1986). Intraradical hyphae stained faintly in trypan blue and no typical arbuscules and vesicles were observed.
PHYLOGENETIC POSITION. Although unknown, suggested to be within the genus Appendicispora belonging in the order Archaeosporales (Spain et al. 2006).
DISTRIBUTION. The holotype of A. myriocarpa comes from spores produced in the culture C-7 with P. phaseoloides as the host plant grown at the Centro Internacional de Agricultura Tropical (CIAT), Cali, Colombia (Schenck et al. 1986). The spores used to establish this culture were originally extracted from native grasses growing in an acid loam (pH 4.5) at Reserva-Carimagua, Meta, Colombia. The authors of the same paper informed that this fungus was also found in many other regions of Colombia and in two sites in Tarapoto, San Martin, Peru, where it has been associated with roots of tropical grasses and legumes, as well as with roots of Brachyaria sp. (Schenck et al. 1986). Additionally, Dodd et al. (1990) revealed A. myriocarpa among roots of Sorghum sp. growing in acid-infertile soils of the savanna ecosystem in the eastern plains of Colombia, and Gai et al. (2006) reported this fungus from China.
NOTES. The morphological and biochemical properties of spores and mycorrhizae of A. myriocarpa defined above come from the combination of the data originally published by Schenck et al. (1986) and results of J. Blaszkowski's observations of specimens of this fungus (a vial C-7 CIAT 404 18.II.86 with aggregates of spores immersed in lactic acid) provided by Dr. E. Sieverding, Institute for Plant Production and Agroecology in the Tropic and Subtropics, University of Hohenheim, Germany. All the spores examined were clustered in loose aggregates. These spores were well preserved, but none either was associated with a sporiferous saccule or had a pedicel.
The presence of the inner germinal wall layer 2 in spores of A. myriocarpa, originally described as a spore wall 3 (Schenck et al. 1986), was here accepted, although examination of crushed spores loaned from Dr. E. Sieverding revealed it in only two specimens. Schenck et al. (1986) emphasized this layer to be very thin (<0.3 µm) and visible only in spores stained in trypan blue or cotton blue. The suggested reason of the increased visibility of this layer was its enhanced thickness due to the deposition of stain particles on its surfaces.
The description of A. myriocarpa presented here is somewhat modified compared with that prepared by Schenck et al. (1986). First, the term “sporocarps” used in the original description of this species was replaced with the term “aggregates”. Morton and Benny (1990) defined sporocarps as structures containing spores embedded within compact intertwining hyphae or around a central hyphal plexus. Meanwhile, spores of A. myriocarpa occur in loose conglomerations without a peridium. Second, the three walls of spores of A. myriocarpa located by Schenck et al. (1986) in one group sensu Walker (1983) are three layers of two walls here, a spore wall and an inner germinal wall. Third, the first layer of the spore wall and layer 1 of the 2-layered inner germinal wall of A. myriocarpa corresponding with the spore walls 1 and 2, respectively, in Schenck's et al. (1986) description are slightly flexible and do not break in even vigorously crushed spores. Thus, they are semi-flexible layers sensu Morton (2002), and not rigid as originally defined (Schenck et al. 1986). Forth, examination of spores by the author of this website showed that the wall layer of spores forming their surface is associated with a flexible, colourless, thin layer adherent to its lower surface. In vigorously crushed spores, this layer usually slightly separated from layer 1 and only occasionally adhered to the upper surface of the first layer of the inner germinal wall. This layer, considered here a spore wall layer 2, has been omitted in the original description of A. myriocarpa (Schenck et al. 1986).
Additionally, in some provided specimens of A. myriocarpa, highly deteriorated fragments of an unidentified structure were found on the surface of their structural spore wall layer (layer 1). These fragments may have been remnants of an evanescent layer forming the spore surface of almost all known species of arbuscular fungi producing acaulosporioid spores, in which it also is a component of the wall of the sporiferous saccule (e. g., Blaszkowski 2003; Morton 2002). Although this layer was not included in the description of the spore wall structure of A. myriocarpa presented above, its putative presence should be confirmed in further studies of this fungus.
The only other species of the genus Acaulospora whose spores are grouped in conglomerations resembling aggregates of A. myriocarpa are A. sporocarpia and A. thomii. Apart from this character, the former species is completely unlike the latter two fungi. First, spores of A. myriocarpa are hyaline and small [(22.0-)32-90 µm diam when globose; Blaszkowski, pers. observ.; Schenck et al. 1986], and those of A. sporocarpia and A. thomii are coloured [dark brown to black and brownish orange (6C8) to brown (6E8), respectively] and large [(140-)160-200(-240) x (-240) x (125-)150-175(-200) µm and (150-)185(-240) µm diam when globose, respectively; Berch 1985; Blaszkowski 2003]. Second, the subcellular structure of spores of A. myriocarpa consists of two 2-layered walls (Blaszkowski, pers. observ.). In contrast, spores of A. sporocarpia comprise only two 1-layered walls, and those of A. thomii have a 3-layered spore wall and two 2-layered inner germinal walls (Blaszkowski 2003). Finally, the spore aggregates of A. sporocarpia are much larger (2.5 x 1.5 x 1.5 cm; Berch 1985) than those of A. myriocarpa [725(-1050) x 525(-906) µm].
Other arbuscular fungi producing colourless acaulosporioid spores of a similar size range to that of A. myriocarpa are A. polonica and Archaeospora trappei. Morphologically, these fungi markedly differ in the number and phenotypic properties of the components of their spores. While spores of A. myriocarpa contain two 2-layered walls, a spore wall and an inner germinal wall, spores of A. polonica are composed of a 2-layered spore wall and two inner germinal walls (Blaszkowski 2003). Spores of the former fungus do not possess the first 1-layered germinal wall of the latter species. Additionally, the structural layer 1 of the spore wall of A. myriocarpa is uniform, and that of A. polonica is laminate.
Similarly as spores of A. myriocarpa, those of Ar. trappei include two walls. However, the outer spore wall layer continuous with the wall of the sporiferous saccule neck of Ar. trappei deteriorates with age (Blaszkowski 2003; Morton 2002; Morton and Redecker 2001), and that of A. myriocarpa is persistent as Schenck et al. (1986) described. Moreover, the inner germinal wall of the latter fungus is much thicker (up to 4.9 µm; Blaszkowski 2003) than that of the former species (up to 1.8 µm; Schenck et al. 1986).
When not associated with a sporiferous saccule, single spores of A. myriocarpa also resemble spores of Intraspora schenckii. Their subcellular structure is identical to that of Ar. trappei (Blaszkowski 2003; Sieverding and Oehl 2006). However, spores of I. schenckii do not form laterally on the neck of a sporiferous saccule as do those of Ar. trappei, but inside it as spores of members of the genus Entrophospora (Blaszkowski 2003; Sieverding and Oehl 2006).
When observed under low magnifications, spores of A. myriocarpa with attached pedicels may also be confused with Glomus spp. forming small and hyaline spores, as Gl. diaphanum, Gl. minutum, Gl. viscosum, Par. laccatum, and Par. occultum. Examination of the subcellular structure of spores of these fungi readily separates them. None of the Glomus spp. listed above has the inner germinal wall of A. myriocarpa (Blaszkowski 2003; Morton 2002; Renker et al., in press).
The lack of results of molecular analyses of A. myriocarpa makes impossible to confirm its relationship with the species producing acaulosporioid spores compared here. However, the lack in A. myriocarpa spores of both a beaded layer and a plastic layer present in the innermost germinal wall of most known Acaulospora spp. and the faint staining of mycorrhizal structures of A. myriocarpa (Schenck et al. 1986) link this fungus with members of the family Archaeosporaceae of the order Archaeosporales (Morton and Redecker 2001). Moreover, the formation of spores at the tip of a pedicel branched from the neck of the sporiferous saccule suggests its affiliation to the recently proposed new genus in this family, i. e., Appendicispora, whose name comes from the combination of two Latin terms: “appendici -“ (prefix from “appendix”, appendix or pedicel) and “spora” (spore; Spain et al. 2006).
REFERENCES
Berch S. M. 1985. Acaulospora sporocarpia, a new sporocarpic species, and emendation of the genus Acaulospora (Endogonaceae, Zygomycotina). Mycotaxon 23, 409-418.
Blaszkowski J. 2003. Arbuscular mycorrhizal fungi (Glomeromycota), Endogone, and Complexipes species deposited in the Department of Plant Pathology, University of Agriculture in Szczecin, Poland. http://www.agro.ar.szczecin.pl/~jblaszkowski/.
Dodd J. C., Arias I., Koomen I., Hayman D. S. 1990. The management of populations of vesicular-arbuscular mycorrhizal fungi in acid-infertile soils of a savanna ecosystem. II. The effect of pre-crops on the spore populations of native and introduced VAM-fungi. Plant and Soil 122, 241-247.
Gai J. P., Christie P., Feng G., Li X. L. 2006. Twenty years of research on biodiversity and distribution of arbuscular mycorrhizal fungi in China: a review. Mycorrhiza 16, 229-239.
Morton J. B. 2002. International Culture Collection of (Vesicular) Arbuscular Mycorrhizal Fungi. West Virginia University: http://www.invam.caf.wvu.edu/.
Morton J. B., Benny G. L. 1990. Revised classification of arbuscular mycorrhizal fungi (Zygomycetes): a new order, Glomales, two new suborders, Glomineae and Gigasporineae, and two new families, Acaulosporaceae and Gigasporaceae, with an emendation of Glomaceae. Mycotaxon 37, 471-491.
Morton J. B., Redecker D. 2001. Two families of Glomales, Archaeosporaceae and Paraglomaceae, with two new genera Archaeospora and Paraglomus, based on concordant molecular and morphological characters. Mycologia 93, 181-195.
Renker C., Blaszkowski J., Buscot F. Paraglomus laccatum comb. nov. – a new member of Paraglomeraceae (Glomeromycota). Nova Hedwigia (in press).
Schenck N. C., Spain J. L., Sieverding E. 1986. A new sporocarpic species of Acaulospora (Endogonaceae). Mycotaxon 25, 111-117.
Sieverding E., Oehl F. 2006. Revision of Entrophospora and description of Kuklospora and Intraspora, two new genera in the arbuscular mycorrhizal Glomeromycetes. J. Appl. Bot. Food Qual. 80, 69-81.
Spain J. L., Sieverding E., Oehl F. 2006. Appendicispora: a new genus in the arbuscular mycorrhiza-forming Glomeromycetes, with a discussion of the genus Archaeospora. Mycotaxon 97, 163-182.
Walker C. 1983. Taxonomic concepts in the Endogonaceae: spore wall characteristics in species descriptions. Mycotaxon 18, 443-455.