GERMINATION.
Unknown.
MYCORRHIZAE.
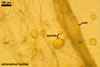
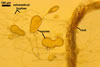
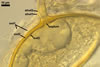
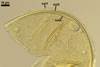
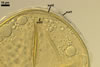
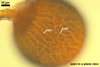
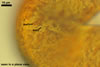
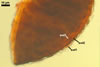
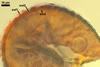
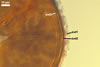
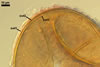
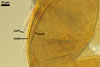
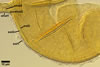
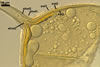
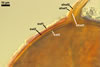
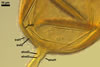
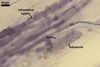
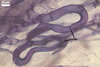
In the field, Gl. hoi has been associated with arbuscular mycorrhizae of plants listed in the section “Distribution and habitat” (see below). In a one-species culture with Plantago lanceolata L. as the host plant, Gl. hoi formed mycorrhizae consisting of arbuscules, as well as intra- and extraradical hyphae. No vesicle was found in the root fragments examined. Arbuscules were not numerous and unevenly dispersed along the roots. They consisted of short trunks grown from parent hyphae and numerous branches with fine tips. Intraradical hyphae grew along the root axis, were (2.2-)7.4(-13.2) µm wide, straight or slightly recurved, and sometimes formed H- or Y-shaped branches and coils. The coils usually were ellipsoid; 15.0-32.5 x 42.5-190.0 µm; when seen in a plane view. Extraradical hyphae were (3.4-)6.2(-11.3) µm wide and occurred not abundantly to very abundantly, depending on the root fragment considered. In 0.1% trypan blue, arbuscules stained violet white (16A2) to pastel violet (16A4), intraradical hyphae pastel violet (16A4) to reddish violet (16B8), coils pale violet (16A3) to deep violet (16D8), and extraradical hyphae deep violet (16D8-E8).
|
|
|
|
|
|
|
|
|
In roots of P. lanceolata |
PHYLOGENETIC POSITION. Unknown.
DISTRIBUTION. The holotype of Gl. hoi has been selected from spores extracted from a pot culture with Zea mays L. as the host plant inoculated with roots of Fragaria chiloensis (L.) Duch. collected from Oregon, Linn Co., Tombstone Pass. on 14 Sept. 1969 (Berch and Trappe 1985). The same scientists also found this fungus in other pot cultures with transplanted plants of dunes, forests, and roadsides [Rubus ursinus Cham. & Schlecht., Epilobium watsonii Barb. & in Brew. & Wats., Maianthemum dilatatum (Wood) Nels. & Macbr., Stachys mexicana Benth., Lolium sp.] of British Columbia, Canada, as well as Oregon and Washington, U.S.A. Other known sites of the occurrence of Gl. hoi are, e. g., sandy soils of Iceland (Greipsson et al. 2002), a cultivated field at the MTT Laukaa Research and Elite Plant Station in Central Finland (Vestberg et al. 2005), and an uncharacterized site located in China (Gai et al. 2006).
NOTES. Glomus hoi characterized here was extracted from a part of a one-species pot culture grown by Dr. Mauritz Vestberg, MTT Plant Production Research Laukaa, Vihtavuori, Finland. This culture was established on 6 Dec. 2006 from spores obtained from the International Bank for the Glomeromycota (isolate BEG 648), its host plant was P. lanceolata, and the growing substrate was a mixture of Terragreen and a coarse-grained sand (1:1 v/v).
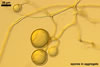
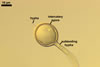
The distinctive properties of Gl. hoi are its spores formed in loose aggregates and the 3-layered spore wall with the mucilaginous outermost layer staining intensively in Melzer's reagent and the flexible to semiflexible innermost one.
Size and colour of spores of Gl. hoi BEG 648, as well as the width of their subtending hyphae and the thickness of their wall generally are within those originally described by Berch and Trappe (1985). In the original description of Gl. hoi, its spore wall has been characterized to consist of two layers, omitting the mucilaginous outermost one revealed in the study discussed here. Although this layer is visible in all the spores illustrated, it was considered an upper part of the laminate one. Not included in the protologue is the staining reaction of this layer. This probably was the main reason of the omission of this layer.
Spores of two groups of species more or less resemble those of Gl. hoi. The species of the first group forming loose aggregates of spores whose wall comprises a flexible innermost layer are Gl. antarcticum M. Cabello, Gl. bagyarajii V.S. Mehrotra, Gl. claroideum N.C. Schenck & G.S. Sm., Gl. dimorphicum Boyetchko & J.P. Tewari, Gl. fasciculatum (Thaxt.) Gerd. & Trappe emend. C. Walker & Koske, and Gl. glomerulatum Sieverd.
Compared with Gl. hoi, Gl. antarcticum produces much larger spores [50-70 µm diam vs. (50-)80-120(-155) µm diam when globose] and they occur in more compact aggregates (Cabello et al. 1995). In Gl. hoi, the outermost spore wall layer stains intensively in Melzer's reagent, whereas none of the components of the spore wall of the latter species reacts in this reagent.
The number and phenotypic and biochemical properties of the spore wall components mainly separate Gl. hoi and Gl. bagyarajii. While the spore wall of Gl. hoi consists of three layers, that of Gl. bagyarajii is 4-layered (Mehrotra 1997). The spore wall component of Gl. bagyarajii not occurring in the spore wall structure of G. hoi is the third unit layer.
Considering spores of Gl. claroideum, those of the fungus characterized here are slightly smaller [(50-)80-120(-155) µm diam vs. (95-)135(-190) µm diam when globose] and usually are grouped in aggregates (vs. usually single in the soil in Gl. claroideum; Blaszkowski 2003; Morton 2002; Schenck and Smith 1982; Stürmer and Morton 1997). Most importantly, the spore wall of the former species comprises four layers, and not three as in the latter fungus. Glomus hoi does not differentiate the second semipermanent, semiflexible spore wall layer of Gl. claroideum.
In contrast to Gl. hoi producing only one type of spores, G. dimorphicum forms two spore morphotypes, one in aggregates and another singly in the soil (Boyetchko and Tewari 1986). Although spores of the latter species occurring in aggregates highly resemble in colour and size those of the former fungus, their wall is only 2-layered, lacking the mucilaginous spore wall layer 1 of Gl. hoi. The second morphotype of Gl. dimorphicum is represented by spores of a wall similar in structure and the phenotypic characters of its components to those of Gl. hoi. However, the spores may be much larger (up to 300 µm diam vs. up to 155 µm diam in Gl. hoi when globose). Unfortunately, in the original description of Gl. dimorphicum, there is no mention of the biochemical properties of its spores. Thus, it is impossible to compare this taxonomically important property in the species considered.
Spores of Gl. fasciculatum and Gl. hoi are similar in colour and size (Blaszkowski 2003; Walker and Koske 1987). However, considering the properties of the components of their spore wall, these species share only their flexible innermost layer. The outermost spore wall layer of Gl. fasciculatum is permanent and does not slough with age as does that of Gl. hoi. Although the middle layer of the 3-layered spore wall of both species is laminate, it stains intensively in Melzer's reagent in Gl. fasciculatum, a phenomenon not occurring in Gl. hoi and rarely found in other known Glomus spp. (Blaszkowski, pers. observ.). Additionally, this layer is much thicker in Gl. fasciculatum [(2.0-)8.5(-16.0) µm thick] than in Gl. hoi [(1.5-)2.2(-3.4) µm thick].
Four characters separate Gl. glomerulatum and Gl. hoi. First, the aggregates of spores of Gl. glomerulatum are more compact than those of Gl. hoi (Blaszkowski 2003; Sieverding 1987). Second, spores of the former species origin only intercalary and, hence, all have two subtending hyphae (vs. mainly terminally and with one subtending hypha in Gl. hoi). Third, spores of Gl. glomerulatum are much smaller (40-70 µm diam when globose) compared with those of Gl. hoi [(50-)80-120(-155) µm diam] and their wall comprises only two layers, a laminate outer layer and a flexible inner one. Forth, even the upper range of width of the Gl. glomerulatum subtending hypha (7 µm wide) does not attain the lower one of that of Gl. hoi (9.3 µm wide).
The second group, mentioned at the beginning of this section, is represented by Gl. aggregatum N.C. Schenck & G.S. Sm. emend. Koske and Gl. intraradices N.C. Schenck & G.S. Sm. Both species produce yellow-coloured spores in aggregates and singly in the soil (Blaszkowski 2003; Morton 2002; Schenck and Smith 1982; Stürmer and Morton 1997). They also are of a size similar to that of Gl. hoi spores, but their wall does not contain the flexible innermost spore wall layer of this fungus. Additionally, the unique property of Gl. aggregatum is the formation of inner spores by internal proliferation.
REFERENCES
Berch S. M., Trappe J. M. 1985. A new species of Endogonaceae, Glomus hoi. Mycologia 77, 645-657.
Blaszkowski J. 2003. Arbuscular mycorrhizal fungi (Glomeromycota), Endogone, and Complexipes species deposited in the Department of Plant Pathology, University of Agriculture in Szczecin, Poland. http://www.agro.ar.szczecin.pl/~jblaszkowski.
Boyetchko S. M., Tewari J. P. 1986. A new species of Glomus (Endogonaceae, Zygomycotina) mycorrhizal with barley in Alberta. Can. J. Bot. 64, 90-95.
Cabello M., Gaspar L., Pollero R. 1994. Glomus antarcticum sp. nov., a vesicular-arbuscular mycorrhizal fungus from Argentina. Mycotaxon 60, 123-128.
Gai J. P., Christie P., Feng G., Li X. L. 2006. Twenty years of research on biodiversity and distribution of arbuscular mycorrhizal fungi in China: a review. Mycorrhiza 16, 229-239.
Greipsson S., El-Mayas H., Vestberg M., Walker C. 2002. Arbuscular mycorrhizal fungi in sandy soils in Iceland. Arctic, Antarctic, Alpine Res. 34, 419-427.
Koske R. E. 1985. Glomus aggregatum emended: A distinct taxon in the Glomus fasciculatum complex. Mycologia 77, 619-630.
Mehrotra V. S. 1997. Glomus bagyarajii sp. nov., a new species of Glomaceae (Glomales, Zygomycees) from India. Philippine J. Sci. 126, 233-242.
Morton J. B. 2002. International Culture Collection of (Vesicular) Arbuscular Mycorrhizal Fungi. West Virginia University. http://www.invam.caf.wvu.edu/.
Schenck N. C., Smith G. S., 1982. Additional new and unreported species of mycorrhizal fungi (Endogonaceae) from Florida. Mycologia 74, 77-92.
Sieverding E. 1987. A VA-mycorrhizal fungus, Glomus glomerulatum sp. nov., with two hyphal attachments and spores formed only in sporocarps. Mycotaxon 29, 73-79.
Stürmer S. L., Morton J. B. 1997. Developmental patterns defining morphological characters in spores of four species in Glomus. Mycologia 89, 72-81.
Vestberg M., Saari K.,Kukkonen S., Hurme T. 2005. Mycotrophy of crops in rotation and soil amendment with peat influence the abundance and effectiveness of indigenous arbuscular mycorrhizal fungi in field soil. Mycorrhiza 15, 447-458.
Walker C., Koske R. E. 1987. Taxonomic concepts in the Endogonaceae: IV. Glomus fasciculatum redescribed. Mycotaxon 30, 253-262.