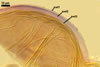
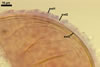
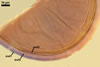
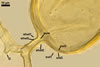
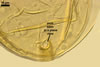
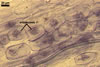
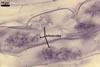
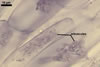
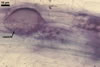
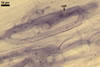
GERMINATION.
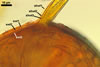
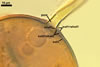
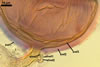
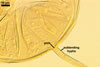
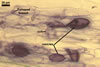
Not observed by the author of this website. According to Morton (2002) and Stürmer and Morton (1997), spores of Gl. intraradices appear to germinate by a germ tube arising from the innermost sublayer (lamina) of the spore wall layer 3. Then, the germ tube emerges from the lumen of the subtending hypha. Additionally, in some specimens, a germ tube arises from broken ends of hyphal fragments some distance from the spore base. This behaviour probably accounts for the high infectivity of hyphal fragments of this species.
MYCORRHIZAE.
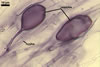
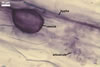
In one-species pot cultures with Plantago lanceolata L. as the host plant, mycorrhizae of Gl. intraradices consisted of arbuscules, vesicles, as well as intra- and extraradical hyphae. Arbuscules were very numerous and evenly distributed along the root fragments examined. They consisted of a trunk grown from a parent hypha and many branches with very fine tips. Vesicles occurred sporadically and were widely dispersed along the axis of the root fragments. They were ellipsoid; 20.0-32.0 x 27.5-60.0 µm. The intraradical hyphae usually extended parallel to the root axis and were (2.0-)4.0(-5.6) µm wide. They sometimes formed Y- or H-shaped branches and frequently coils. The coils were ellipsoid; 15.0-20.0 x 40.0-97.5 µm; rarely circular; 35.0-45.0 µm diam; when observed in a plane view. The extraradical hyphae were (1.7-)2.5(-4.4) µm wide and occurred abundantly. In 0.1% trypan blue, arbuscules stained pale violet (16A3) to royal purple (16D8), vesicles light lilac (16A5) to royal purple (16D8), intraradical hyphae violet white (16A2) to reddish violet (16A8), coils violet white (16A2) to reddish violet (16C8), and extraradical hyphae violet white (15A2) to reddish violet (16A8).
PHYLOGENETIC
POSITION. Results of molecular analyses have accommodated Gl. intraradices in subclade b of Glomus group A in the family Glomeraceae Piroz. & Dalpé of the order Glomerales J.B. Morton & Benny, also comprising Gl. clarum Nicol. & N.C. Schenck, Gl. coremioides (Berk. & Broome) D. Redecker & J.B. Morton, Gl. fasciculatum (Thaxt.) Gerd. & Trappe emend. C. Walker & Koske, Gl. manihotis R.H. Howeler, Sieverd. & N.C. Schenck, Gl. proliferum Dalpe & Declerck, Gl. sinuosum (Gerd. & B.K. Bakshi) R.T. Almeida & N.C. Schenck, and Gl. vesiculiferum (Thaxt.) Gerd. & Trappe (Schwarzott et al. 2001). Unfortunately, the phylogenetic distance between Gl. intraradices and Gl. aggregatum N.C. Schenck & G.S. Sm. emend. Koske, Gl. antarcticum M. Cabello, Gl. aureum Oehl & Sieverd., Gl. cerebriforme McGee, Gl. glomerulatum Sieverd., Gl. invermaium I.R. Hall, and Gl. pallidum I.R. Hall, species compared below as well, remains unknown to date.
DISTRIBUTION.
In Poland, the author of this website found spores of Gl. intraradices in 25 samples of rhizosphere soils and roots. Of them, five came from under Ammophila arenaria (L.) Link, Artemisia campestris L. and Petasites spurius (Retz.) Rchb. colonizing maritime sand dunes adjacent to Swinoujscie (53º55'N, 14º14'E) in October 1993 and June 1997, three from under P. lanceolata growing in the Tuchola Forest (53º46'N, 17º42'E-53º40'N, 17º54'E) in September 1996, four from under Festuca rubra L. s. s. and Holcus mollis L. colonizing the inland sand dunes of the Bledowska Desert (50o22'N, 19o34'E) in June 1997, five from under A. arenaria and Agrostis stolonifera L. growing in maritime sand dunes of the Slowinski National Park (54o45'N, 17o26'E) in August 1996, one from under
Hieracium sp. growing in an arsenic heap near Zloty Stok (50º26'N, 16º52'E) and sampled in July 2002, four from under Taxus baccata L. growing in the Central Cemetery in Szczecin (53º26'N, 14º35'E) in May 2001, and four from under Juncus conglomeratus L. em. Leers colonizing the bank of the Puck Bay (54º42'N, 18º28'E) in August 2001.
Additionally, Turnau et al. (2001) detected Gl. intraradices in roots of Fragaria vesca L. colonizing a 20-year-old Zn waste located in Chrzanów (50º08'N, 19º24'E) in southern Poland using a nested polymerase chain reaction with taxon-specific primers, although no spores of this fungus were found.
The holotype of Gl. intraradices has been selected from spores extracted from pot-cultured Paspalum notatum Flugge initiated from a sample originally isolated from among roots of Citrus sp. cultivated in Orlando, Florida, U.S.A. (Schenck and Smith 1982). Schenck and Smith (1981, 1982) found this species to be one of the most common Glomus species occurring in Florida, where it was associated with roots of many plant species.
Literature data and investigations of the author of this website indicate Gl. intraradices to have a worldwide distribution. Apart from Poland and Florida, this fungus has also been encountered in many other regions of the U.S.A., e. g., in California (Bethlenfalvay et al. 1984; Koske and Halvorson 1989), Kentucky (An et al. 1993),
Massachusetts (Blaszkowski, unpubl. data), Texas (Stutz and Morton 1996) and Hawaii (Koske and Gemma 1996), as well as in Canada (Dalpé 1989; Klironomos et al. 2001), Portugal (Blaszkowski, unpubl. data), Bornholm (Denmark; Blaszkowski, unpubl. data), Switzerland (Jansa et al. 2002; Oehl et al. 2005), Africa (Blaszkowski, unpubl. data; Stutz et al. 2000), Israel (Blaszkowski and Czerniawska 2006), Turkey and Cyprus (Blaszkowski, unpubl. data), China (Gai et al. 2006; Zhang and Wang 1992), and India (Mohankumar et al. 1988).
Blaszkowski's investigations of field-collected mixtures of rhizosphere soils and roots and those sampled from trap cultures established from a part of the field mixtures revealed that the arbuscular fungi co-occurring with Gl. intraradices were Acaulospora lacunosa J.B. Morton, A. mellea Spain & N.C. Schenck, A. morrowiae Spain & N.C. Schenck, Archaeospora trappei (R.N. Ames & Linderman) J.B. Morton & D. Redecker emend. Spain, Entrophospora baltica Blaszk., Madej & Tadych, Gl. aggregatum, Gl. arenarium Blaszk., Tadych & Madej, Gl. clarum, Gl. constrictum Trappe, Gl. corymbiforme Blaszk., Gl. deserticola Trappe, Bloss & J.A. Menge, Gl. etunicatum W.N. Becker & Gerd., Gl. fasciculatum, Gl. gibbosum Blaszk., Gl. insculptum Blaszk., Gl. macrocarpum Tul. & C. Tul., Gl. microcarpum Tul. & C. Tul., Gl. lamellosum Dalpé, Koske & Tews, Gl. pustulatum Koske, Friese, C. Walker & Dalpé, an undescribed Glomus sp., Paraglomus laccatum (Blaszk.) C. Renker, Blaszk. & F. Buscot, Scutellospora armeniaca Koske & Halvorson, and S. dipurpurescens J.B. Morton & Koske.
The spore abundance of Gl. intraradices in the field samples ranged from 1 to 30 in 100 g dry soil, and the proportion of spores of this fungus in spore populations of all the arbuscular fungi isolated ranged from 0.7 to 33.3%.
NOTES. When observed under a dissecting microscope, three groups of species of the genus Glomus known to form spores both singly and in aggregates more or less resemble Gl. intraradices. The group of species most similar in colour and size of spores to those of Gl. intraradices is represented by Gl. aggregatum, Gl. antarcticum, Gl. fasciculatum, Gl. pallidum, Gl. proliferum, and G. vesiculiferum.
Considering the phenotypic and biochemical properties of the components of the spore wall of these species observed under a light microscope, Gl. intraradices is most closely related to Gl. aggregatum. The number and the types of layers forming the spore wall of these species and their reactivity in Melzer's reagent are identical (Blaszkowski 2003; pers. observ.). The only character distinguishing these fungi is the formation of spores inside of their parent spores by internal proliferation in Gl. aggregatum (Koske 1985), a phenomenon not found in Gl. intraradices (Blaszkowski, pers.observ.; Stürmer and Morton 1997). Morton (2002) hypothesized these species to be synonymous. Thus, results of molecular analyses of both fungi are urgently needed to explain this supposition.
Similarly as in Gl. intraradices, the spore wall of Gl. antarcticum and Gl. fasciculatum is 3-layered (Blaszkowski 2003; Cabello et al. 1994; Walker and Koske 1987). However, of these layers of Gl. antarcticum, only the outermost one, forming the spore surface, sloughs with age. In contrast, in Gl. intraradices, two outer spore wall layers are of the type of sloughing layers. Moreover, the spore wall of Gl. intraradices lacks the innermost, flexible layer of the Gl. antarcticum spore wall. Finally, while the outermost spore wall layer of Gl. intraradices stains intensively in Melzer's reagent, none of the wall layers of Gl. antarcticum spores reacts in this reagent.
As mentioned above, Gl. fasciculatum also produces spores of a 3-layered wall, of which all are permanent, however (Blaszkowski 2003; Walker and Koske 1987; vs. two outer layers slough with age in Gl. intraradices). Similarly as in Gl. antarcticum, the distinctive component of the spore wall of Gl. fasciculatum is an innermost flexible, colourless layer, which is lacking in the wall of spores of Gl. intraradices. Still other important difference between these fungi regards the reactivity of their spores in Melzer's reagent. While the structure of spores of Gl. intraradices staining in this reagent is only their outermost spore wall layer, two outer wall layers of spores of Gl. fasciculatum are reactive in Melzer's reagent, including its laminate layer, not staining in any other known species of the genus Glomus (Blaszkowski, pers. observ.). Additionally, Gl. intraradices probably is much more plastic ecologically than Gl. fasciculatum. The former fungus has successfully been used in many experiments (Gopi et al. 2000). Although Gl. fasciculatum has been one of the most frequently cited species of arbuscular fungi in papers describing the influence of arbuscular fungi on plants, Walker and Koske (1987) concluded this fungus to had certainly been confused with other species of the Glomeromycota. Many attempts to grow Gl. fasciculatum in one-species cultures made by the author of this website failed. In the literature, there is no convincing evidence of the properties of mycorrhizae of Gl. fasciculatum from a one-species culture.
Although Gl. proliferum has originally been described to form hyaline spores (Declerck et al. 2000), pictures obtained from Dr. C. Walker, U. K., also show yellow-coloured spores of this fungus, deceptively similar to those of Gl. intraradices. However, in respect of size, only the largest spores of the former species attain the lower size range of spores of the latter fungus (Blaszkowski, pers. observ.; Declerck et al. 2000). The spore wall of Gl. proliferum has originally been characterized to consist of four permanent layers, but examination of this fungus (culture: MVCL 41827) obtained from Prof. S. Declerck, Université catholique de Louvain, Mycothèque de l'Université catholique de Louvain, Unite de microbiologie, Belgium, revealed only three layers of phenotypic and biochemical properties identical to those of Gl. intraradices.
At least three morphological characters separate Gl. intraradices and Gl. pallidum. First, Hall (1977) characterized spores of the latter species to be whitish, and not yellow-coloured as most mature spores of the former fungus. Second, spores of Gl. pallidum generally are smaller than those of Gl. intraradices [32-78 x 28-68 µm diam according to Hall 1977; vs. (30-)92(-120) µm diam or (60-)80-120(-160) µm diam as the author of this website and Stürmer and Morton (1997) determined, respectively].Third, in contrast to the 3-layered spore wall of Gl. intraradices, only two layers build the spore wall of Gl. pallidum. Among them, the middle, semi-flexible wall layer of Gl. intraradices spores is lacking.
The unique structures of Gl. vesiculiferum are its thin-walled vesicles associated with a peridium-like layer covering sporocarps of this fungus (Gerdemann and Trappe 1974). Additionally, spores of Gl. vesiculiferum generally are smaller (49-85 µm diam when globose or up to 100 x 70 µm when ovoid to irregular; Gerdemann and Trappe (1974) than those of Gl. intraradices (see above) and have only a 2-layered wall (Gerdemann and Trappe 1974 vs. 3-layered in Gl. intraradices).
The second group of species compared here represents only Gl. cerebriforme, whose spores partly overlap in size with those of Gl. intraradices [25 x 25-65 x 80 µm diam after McGee (1986) vs. (30-)92(-120) µm diam or (60-)80-120(-160) µm diam according to the author of this website and Stürmer and Morton (1997), respectively], but remain hyaline throughout their entire life cycle, and, thereby, are similar only to the juvenile spores (Blaszkowski, pers. observ.) of the species discussed here. Moreover, the distinctive character of the former species is the formation of its spores on racemose hyphae, and not on hyphae irregularly branched as in the latter fungus.
The next diametrical differences between these species occur in the number, the phenotypic characters, and the spatial distribution of layers of their spore wall. The spore wall of Gl. cerebriforme consists of a thick, laminate outer layer and a thin, flexible inner one (McGee 1986). Thus, the structural layer of this wall is an outermost layer, forming the spore surface, and not an innermost layer as in the spore wall of Gl. intraradices, which is covered with two impermanent layers, but does not overly a flexible layer as in Gl. cerebriforme. Additionally, compared with Gl. intraradices, the subtending hypha of Gl. cerebriforme spores is much narrower [3-7 µm wide after McGee (1986) vs. (13.7-)15.5(-18.4) µm wide as presented here].
The third group of species superficially resembling Gl. intraradices comprises Gl. aureum, Gl. glomerulatum, and Gl. invermaium. Compared with the relatively large (92-120 µm diam when globose) and pale yellow (3A3) to greyish yellow (2B5) mature spores of Gl. intraradices (Blaszkowski, pers. observ.), globose spores of all the other species are smaller and darker-coloured [(27-)40-60 µm diam, light orange (5A4) to orange (5A7) in Gl. aureum; Blaszkowski, pers. observ.; Oehl et al. 2003; 40-70 µm diam, light orange (5A5) to golden yellow (5B8) in Gl. glomerulatum; Blaszkowski, pers. observ.; Sieverding 1987; 50-75 µm diam, light brown to brown in Gl. invermaium; Hall 1977]. Except for Gl. intraradices having a 3-layered spore wall, that of all these species is 2-layered. Moreover, the layers forming the spore surface of Gl. glomerulatum and Gl. invermaium are laminate and unit sensu Walker (1983), respectively, thus, they are permanent, whereas the outermost spore wall layer of Gl. intraradices lives shortly and usually is completely sloughed or at most occurs patchily as a highly decomposed structure at maturity.
The distinctive character of spores of Gl. glomerulatum also is that the innermost layer of their wall is a thin, flexible, membranous, uniform structure, and not a rigid layer composed of many sublayers (laminae) as in the other three species and that Gl. glomerulatum produces only intercalary spores, which, thereby, always have two subtending hyphae (Blaszkowski, pers. observ.; Sieverding 1987). An intercalary mode of spore origination has also been observed in Gl. intraradices and many other species of the Glomeromycota (Blaszkowski, pers. observ.), but such spores usually constituted a small part of all the spores produced.
The last morphological character distinguishing species of this group is the width of the subtending hyphae of their spores. It is widest in Gl. intraradices [(13-7)15.5(-18.4) µm wide; Blaszkowski, pers. observ.], intermediate in Gl. invermaium (6-13 µm wide; Hall 1977), and narrowest in Gl. glomerulatum (6-10 µm wide; Blaszkowski, pers. observ.; Sieverding 1987).
As presented in the section “Phylogenetic position”, apart from species compared above, Gl. intraradices is molecularly also related to Gl. clarum, Gl. coremioides, Gl. manihotis, and Gl. sinuosum (Schwarzott et al. 2001).
Although there is no formal decision, morphological characters and results of molecular analyses of spores of Gl. clarum and Gl. manihotis have suggested these fungi to be synonymous (Morton 2002; Schwarzott et al. 2001). Morphologically, Gl. clarum differs markedly from Gl. intraradices in colour and size of spores, as well as in phenotypic properties of the components of their wall. Spores of the former fungus may be darker [to yellow-brown; Morton 2002; vs. pale yellow (3A3) to greyish yellow (2B5) or hyaline to greenish yellow after Blaszkowski, pers. observ. and Stürmer and Morton 1997, respectively], much larger when globose [(120-)180-200(-280) µm diam; Stürmer and Morton 1997; vs. (30-)92(-120) µm diam or (60-)80-120(-160) µm diam after Blaszkowski, pers. observ. and Stürmer and Morton 1997, respectively], and have two laminate layers in their 3-layered wall (vs. only one such layer in Gl. intraradices).
Glomus coremioides and Gl. sinuosum are morphologically completely unlike Gl. intraradices. The former two fungi produce compact sporocarps with a peridium (Blaszkowski, pers. observ.; Gerdemann and Trappe 1974; Morton 2002; vs. single spores or in loose aggregates without a peridium in Gl. intraradices), in which spores are organized in a single layer and develop from a central plexus of hyphae (vs. randomly distributed spores when in aggregates and develop terminally from branched hyphae). Moreover, spores of the former two species are (1) ovoid to clavate (vs. usually globose to subglobose in Gl. intraradices), (2) darker-coloured [brown and orange-brown, respectively, after Gerdemann and Trappe 1974 and Morton 2002, respectively; vs. pale yellow (3A3) to greyish yellow (2B5) in Gl. intraradices; Blaszkowski, pers. observ.], and (3) their wall consists of only one layer (Blaszkowski, pers. observ.; Gerdemann and Trappe 1974; Morton 2002; vs. 3-layered in Gl. intraradices).
REFERENCES
An Z.-Q, Hendrix J. W., Hershman D. E., Ferriss R. S., Henson G. T. 1993. The influence of crop rotation and soil fumigation on a mycorrhizal fungal community associated with soybean. Mycorrhiza 3, 171-182.
Bethlenfalvay G. J., Dakessian S., Pacovscky R. S. 1984. Mycorrhizae in a southern California desert: ecological implications. Can. J. Bot. 62, 519-524.
Blaszkowski J. 2003.
Arbuscular mycorrhizal fungi (Glomeromycota), Endogone and Complexipes
species deposited in the Department of Plant Pathology, University of Agriculture
in Szczecin, Poland. http://www.agro.ar.szczecin.pl/~jblaszkowski/.
Blaszkowski J., Czerniawska B. 2006. The occurrence of arbuscular mycorrhizal fungi of the phylum Glomeromycota in Israeli soils. Acta Soc. Bot. Pol. 75, 339-350.
Cabello M., Gaspar L., Pollero R. 1994. Glomus antarcticum sp. nov., a vesicular-arbuscular mycorrhizal fungus from Argentina. Mycotaxon 60, 123-128.
Dalpé Y. 1989. Inventaire et repartition de la flore endomycorhizienne de dunes et de rivages maritimes du Quebec, du Nouveau-Brunswick et de la Nouvelle-Ecosse. Naturaliste can. (Rev. Ecol. Syst.) 116, 219-236.
Declerck S., Cranenbrouck S., Dalpé Y., Séguin S., Grandmougin-Ferjani A., Fontaine J., Sancholle M. 2000. Glomus proliferum sp. nov.: a description based on morphological, biochemical, molecular and monoxenic cultivation data. Mycologia 92, 1178-1187.
Gai J. P., Christie P., Feng G., Li X. L. 2006. Twenty years of research on biodiversity and distribution of arbuscular mycorrhizal fungi in China: a review. Mycorrhiza 16, 229-239.
Gerdemann J. W., Trappe J. M. 1974. The Endogonaceae in the Pacific Northwest. Myc. Memoir 5, 1-76.
Gopi K., Douds P, Douds D. 2000. Current advances in mycorrhizae research. APS Press. The American Phytopathological Society. St. Paul, Minnesota.
Hall I. R. 1977. Species and mycorrhizal infections of New Zealand Endogonaceae. Trans. Br. Mycol. Soc. 68, 341-356.
Jansa J., Mozafar A., Anken T., Ruh R., Sanders I. R., Frossard E. 2002. Diversity and structure of AMF communities as affected by tillage in a temperate soil. Mycorrhiza 12, 225-234.
Klironomos J. N., Hart M. M., Gurney J. E., Moutoglis P. 2001. Interspecific differences in the tolerance of arbuscular mycorrhizal fungi to freezing and drying. Can. J. Bot. 79, 1161-1166.
Koske R. E. 1985. Glomus aggregatum emended: A distinct taxon in the Glomus fasciculatum complex. Mycologia 77, 619-630.
Koske R. E., Gemma J. N. 1996. Arbuscular mycorrhizal fungi in Hawaiian sand dunes: Island of Kaua'i. Pacific Sci. 50, 36-45.
Koske R. E., Halvorson W. L. 1989. Mycorrhizal associations of selected plant species from San Miguel island, Channel Islands national Park, California. Pacific Sci. 43, 32-40.
McGee P. A. 1986. Further sporocarpic species of Glomus (Endogonaceae) from South Australia. Trans. Brit. Mycol. Soc. 87, 123-129.
Mohankumar V., Ragupathy S., Nirmala C. B., Mahadevan A. 1988. Distribution of vesicular arbusculr mycorrhizae (VAM) in the sandy beach soils of Madras coast. Cur. Sci.57, 367-368.
Morton J. B. 2002. International Culture Collection of (Vesicular) Arbuscular Mycorrhizal Fungi. West Virginia University: http://www.invam.caf.wvu.edu/.
Oehl F., Sieverding E., Ineichen K., Ris E.-A., Boller T., Wiemken A. 2005. Community structure of arbuscular mycorrhizal fungi at different soil depths in extensively and intensively managed agroecosystems. New Phytol. 165, 273-283.
Oehl F., Wiemken A., Sieverding E. 2003. Glomus aureum, a new sporocarpic arbuscular mycorrhizal fungal species from European grasslands. J. App. Bot. 77, 111-115.
Schenck N. C., Smith G. S. 1981. Distribution and occurrence of vesicular-arbuscular mycorrhizal fungi on Florida agricultural crops. Soil and Crop Sci. Soc. Florida 40, 171-175.
Schenck N. C., Smith G. 1982. Additional new and unreported species of mycorrhizal fungi (Endogonaceae) from Florida. Mycologia 74, 77-92.
Schwarzott D., Walker C., Schüßler A. 2001. Glomus, the largest genus of the arbuscular mycorrhizal fungi (Glomales) is nonmonophyletic. Mol. Phyl. Evol. 21, 190-197.
Sieverding E. 1987. A VA-mycorrhizal fungus, Glomus glomerulatum sp. nov., with two hyphal attachments and spores formed only in sporocarps. Mycotaxon 29, 73-79.
Stutz J. C., Morton J. B. 1996. Successive pot cultures reveal high species richness of arbuscular endomycorrhizal fungi in arid ecosystem. Can. J. Bot. 74, 1883-1889.
Stutz J. C., Copeman R., Martin C. A., Morton J. B. 2000. Patterns of species composition and distribution of arbuscular mycorrhizal fungi in arid regions of southeastern North America and Namibia, Africa. Can. J. Bot. 78, 237-245.
Stürmer S. L., Morton J. B. 1997. Developmental patterns defining morphological characters in spores of four species in Glomus. Mycologia 89, 72-81.
Turnau K., Ryszka P., Gianinazzi-Pearson V., van Tuinen D. 2001. Identification of arbuscular mycorrhizal fungi in soils and roots of plants colonizing zinc wastes in southern Poland. Mycorrhiza 10, 169-14.
Walker C. 1983. Taxonomic concepts in the Endogonaceae: Spore wall characteristics in species descriptions. Mycotaxon 18: 443-455.
Walker C., Koske R. E. 1987. Taxonomic concepts in the Endogonaceae: IV. Glomus fasciculatum redescribed. Mycotaxon 30, 253-262.
Zhang M-Q., Wang Y-S. 1992. Eight species of VA mycorrhizal fungi from Northern China. Acta mycol. Sinica. 2, 258-267.