GERMINATION.
Not observed.
MYCORRHIZAE.
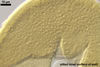
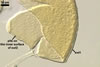
Mycorrhizae of this fungus formed in one-species cultures with
Plantago lanceolata L. as the plant host consisted of arbuscules, intra-
and extraradical hyphae. Arbuscules appeared as granular structures in cortical
cells. Fine branches were difficult to see. Arbuscules were numerous, but unevenly
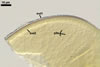
distributed in roots. The intraradical hyphae were (4.2-)6.2(-7.4) µm
wide and grow parallel to the root axis. They were straight or slightly curved,
sometimes dichotomously branched and frequently coiled; the coils were 20.8-34.6
x 10.5-22.3 µm. No vesicles were present in roots of plants up to 8-month-old.
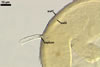
Extraradical hyphae were (1.7-)3.2(-3.5) µm wide and frequently associated
with young and mature spores. In 0.1% trypan blue, arbuscules stained violet
white (17A2), intraradical hyphae violet white (17A2) to pastel violet (17A4),
and extraradical hyphae pale violet (17A3).
DISTRIBUTION.
In Poland, spores of Gl. insculptum have been
revealed in seven field-collected soil samples and 10 trap pot cultures with
rhizosphere soils of five plant species colonizing inland sand dunes of the
Bledowska Desert (50o22’ N, 19o34’ E) located in south of Poland
(Blaszkowski et al., in press). The plant species colonized by Gl. insculptum
in the field have been Corynephorus canescens (L.) P. Beauv., Festuca
rubra L. s. s., Holcus mollis L., Juniperus communis
L., and Plantago major L.
There
is no report of the presence of this fungus in the other regions of both Poland
and the world.
NOTES.
When observed under a dissecting microscope, spores of Gl. insculptum
most resemble small-spored isolates of Gl.
aggregatum N.C. Schenck & G.S. Sm. emend. Koske, Gl.
arenarium Blaszk. at al., Gl. etunicatum W.N. Becker & Gerd.,
Gl. intraradices N.C. Schenck & G.S. Sm., Gl.
pustulatum Koske et al., Gl.
trimurales Koske & Halvorson, and Gl.
versiforme (P. Karsten) S.M. Berch. All eight species form globose to subglobose
and yellow-coloured spores, whose size range partly overlaps (Becker and Gerdemann
1977; Berch and Fortin 1983; Blaszkowski 1991; Blaszkowski et al. 2001, 2003, 2004;
Koske 1985; Koske and Halvorson 1989; Koske et al. 1986; Morton 2000; Schenck
and Smith 1982; Stürmer and Morton 1997).
Examination of subcellular
structure and phenotypic properties of layers in the spore wall of specimens
crushed in Melzer’s reagent readily separates these species. Only Gl.
insculptum forms spores in which the laminate spore wall layer is regularly
pitted. However, in young spores, the inner surface either is smooth or the
pits are very shallow and difficult to see. Even then, Gl insculptum
is distinguishable from other species. Although Gl. etunicatum, Gl.
insculptum, and Gl. versiforme have a spore wall composed of
two layers, the outer layer of each differs. In Gl. etunicatum the
layer sloughs as spores mature (Stürmer and Morton 1997), while in Gl.
versiforme it is semi-permanent (Morton 2000) and in Gl. insculptum
it is permanent. Additionally, the outer spore wall layer of Gl. insculptum
and Gl. versiforme (Morton 2000) is nonreactive in Melzer’s
reagent but stains dark pinkish red to reddish-purple in Gl. etunicatum
(Stürmer and Morton 1997). Glomus versiforme also differs from
Gl. insculptum in the occasional production of spores arranged in
epigeous sporocarps (Berch and Fortin 1983; Morton 2000) vs. only single,
hypogeous spores in Gl. insculptum and in that the mean diameter
of globose spores of the former fungus is almost twice that of spores of the
latter species. In contrast to the two-layered subcellular spore wall structure
of Gl. insculptum, that of Gl. arenarium, Gl. pustulatum,
and Gl. trimurales consists of three layers.
The only other species
of arbuscular fungi forming spores with an ornamented inner surface of their
innermost wall layer are Gl. kerguelense Dalpé &
Strullu and Gl. verruculosum
Blaszk. However, compared with Gl. insculptum, spores of the two
species are much larger (mean diameter = 71 µm in Gl. insculptum
vs. 186.3 µm and 189.0 µm in Gl. kerguelense and Gl.
verruculosum, respectively) and the ornamentation of the innermost layer
of their wall consists of fine granules (Gl. kerguelense) or warts
(Gl. verruculosum; Blaszkowski and Tadych 1997; Dalpé et al.
2002) vs. pits in Gl. insculptum.
REFERENCES
Becker W. N., Gerdemann
J. W. 1977. Glomus etunicatus sp. nov. Mycotaxon 6, 29-32.
Berch S. M., Fortin
J. A. 1983. Lectotypification of Glomus macrocarpum and proposal
of new combinations: Glomus australe, Glomus versiforme,
and Glomus tenebrosum (Endogonaceae). Can. J. Bot. 61, 2608-2617.
Blaszkowski J. 1991.
Polish Endogonaceae. IX. Glomus aggregatum with spores forming an
evanescent outermost wall. Crypt. Bot. 2/3, 130-135.
Blaszkowski J., Tadych
M. 1997. Glomus multiforum and G. verruculosum, two new
species from Poland. Mycologia 89, 804-811.
Blaszkowski J., Adamska
I., Czerniawska B. 2003. Glomus trimurales, an arbuscular mycorrhizal
fungus (Glomerales) new for Poland and Europe. Mycotaxon 87, 425-436.
Blaszkowski J., Adamska
I., Czerniawska B. 2004. Glomus insculptum, a new arbuscular mycorrhizal
species from Poland. Mycotaxon
89, 225-234.
Blaszkowski J., Tadych
M., Madej T. 2001. Glomus arenarium, a new species in Glomales (Zygomycetes).
Acta Soc. Bot. Pol. 70, 97-101.
Dalpé Y., Plenchette
C., Frenot Y., Gloaguen J. C., Strullu D. G. 2002. Glomus kerguelense
sp. nov., a new Glomales species from sub-Antarctic. Mycotaxon 84, 51-60.
Koske R. E. 1985. Glomus
aggregatum emended: A distinct taxon in the Glomus fasciculatum
complex. Mycologia 77, 619-630.
Koske R. E., Halvorson
W. L. 1989. Scutellospora arenicola and Glomus trimurales:
two new species in the Endogonaceae. Mycologia 81, 927-933.
Koske R. E., Friese
C., Walker C., Dalpé Y. 1986. Glomus pustulatum: A new species
in the Endogonaceae. Mycotaxon 26, 143-149.
Morton J. B. 2000. International
Culture Collection of Arbuscular and Vesicular-Arbuscular Mycorrhizal Fungi.
West Virginia University.
Schenck N. C., Smith
G. S. 1982. Additional new and unreported species of mycorrhizal fungi (Endogonaceae)
from Florida. Mycologia 74, 77-92.
Stürmer S. L., Morton
J. B. 1997. Developmental patterns defining morphological characters in spores
of four species in Glomus. Mycologia 89, 72-81.