GERMINATION.
Unknown.
MYCORRHIZAE.
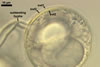
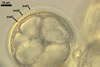
In the field, Gl. proliferum has been associated with roots of Clausena excavata Burm. In one-species pot cultures with Allium porrum L. as the host plant, Gl. proliferum formed mycorrhizae with arbuscules (Declerck et al. 2000).
PHYLOGENETIC
POSITION. According to Schwarzott et al. (2001), Gl. proliferum is a member of the subclade Glomus group Ab (Glomerales J.B. Morton & Benny) also comprising, e. g., Gl. fasciculatum (Thaxt.) Gerd. & Trappe emend. C. Walker & Koske, Gl. intraradices N.C. Schenck & G.S. Sm., and Gl. vesiculiferum (Thaxt.) Gerd. & Trappe. Earlier molecular analyses (Declerck et al. 2000) showed that the most closely related species to Gl. proliferum are Gl. intraradices (97.7% of similarity) and Gl. manihotis R.H. Howeler, Sieverd. & N.C. Schenck (97.1%).
DISTRIBUTION.
So far, Gl. proliferum is only known from a single site at Neufchateau, Capesterre-Belle-Eau, Guadeloupe (16º3'N, 61º37'W; altitude 250 m), France. It was isolated from the rhizosphere of C. excavata growing at the margin of a banana plantation (Declerck et al. 2000).
NOTES. Glomus proliferum presented here was characterized based on spores produced in a monoxenic agar culture established in association with a Ri T-DNA transformed carrot root by Prof. S. Declerck (culture: MVCL 41827), Université catholique de Louvain, Mycothèque de l'Université catholique de Louvain, Unite de microbiologie, Belgium.
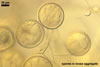
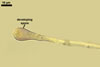
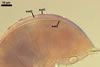
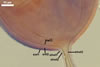
Compared with the original description of Gl. proliferum (Declerck et al. 2000), examination of the spores provided revealed five differences in the phenotypic and biochemical properties of the spore wall components and the subtending hypha, as well as in the spore wall structure. First, the ranges of thickness of wall layers 1 and 2 of the spores studied were slightly wider [layer 1: (1.0-)1.4(-2.0) µm thick, layer 2: (1.5-)2.6(-3.9) µm thick] than those of spores originally characterized [layer 1: 1.0-1.5 µm thick, layer 2: 1.6-2.0 µm thick]. Second, Declerck et al. (2000) defined spore wall layers 1 and 2 to be slightly laminate and unit, respectively, whereas they are mucilaginous (layer 1) and semiflexible and deteriorating with age (layer 2), respectively. Third, all the spores examined by me lacked the membranous innermost spore wall layer 4 revealed by Declerck et al. (2000). This layer probably was a lamina that frequently separates from a laminate layer in vigorously crushed spores (Blaszkowski, pers. observ.). Forth, as results from the original protologue, the spore wall layer 1 does not react in Melzer's reagent. In contrast, this layer in all the spores provided stained intensively in this reagent. Finally, the range of thickness of the subtending hyphal wall of the spores examined also was markedly wider than that given in the original description [(1.7-)3.5(-6.6) µm thick vs. 1.6-3.8 µm thick].
The most distinctive characters of the Gl. proliferum spores provided are its hyaline spores and the relatively thin and compact innermost layer of their 3-layered wall.
Seven other species of the Glomeromycota also form only colourless glomoid spores, i. e., Gl. cerebriforme McGee, Gl. diaphanum J.B. Morton & C. Walker, Gl. minutum Blaszk., Tadych & Madej, Gl. pubescens (Sacc. & Ellis) Trappe & Gerd., Pacispora franciscana Sieverd. & Oehl, Paraglomus laccatum (Blaszk.) C. Renker, Blaszk. & F. Buscot, and Par. occultum (C. Walker) J.B. Morton & D. Redecker.
When observed under a low magnification, only spores of Gl. cerebriforme and Gl. minutum are similar in size to those of Gl. proliferum (Blaszkowski et al. 2000; McGee 1986). Mean and the largest spores of Gl. pubescens are smaller, not attaining the mean size of spores of Gl. proliferum (Blaszkowski 2003).
Spores of the other species compared here may be or are markedly larger than those of Gl. proliferum. Although the largest globose spores of Gl. proliferum (76.0 µ m diam; Declerck et al. 2000) slightly exceed the mean size of spores of Par. occultum (71.5 µ m diam; Morton 2002), they are markedly smaller than the largest spores of the latter fungus (100.0 µm diam). Only the smallest spores of Gl. diaphanum and Par. laccatum (50.0 µm diam) are within the mean size range of spores of Gl. proliferum (40-60 µm diam), whereas mean spores of the two former species are much larger (90.0 µm and 87.0 µm diam, respectively; Blaszkowski 1988, 2003; Renker et al. 2007) than the largest spores of Gl. proliferum (76.0 µm diam). Even the smallest spores of Pac. franciscana (80 µm diam; Blaszkowski 2003) are beyond the upper size range of spores of Gl. proliferum (76.0 µm diam).
Additionally, Gl. cerebriforme spores form on racemose hyphae (McGee 1986; vs. on or in randomly branched hyphae in Gl. proliferum), and those of Gl. pubescens are covered with a peridium (Blaszkowski 2003; Gerdemann and Trappe 1974; vs. no peridium in Gl. proliferum).
Apart from Gl. proliferum, of the fungi listed above, the species forming spores with a 3-layered wall also are Gl. diaphanum, Gl. pubescens, Pac. franciscana, and Par. occultum.
Glomus proliferum and Gl. diaphanum share their mucilaginous outermost layer and the laminate layer of the spore wall (Blaszkowski 2003). However, the mucilaginous wall layer of spores of Gl. proliferum stains more intensively in Melzer's reagent [rose (12A4) to bluish red (12B7)] than that of the spore wall of Gl. diaphanum [pinkish white (7A2)] and the laminate spore wall layer of the former species is the innermost component of this wall, whereas the innermost structure of the 3-layered spore wall of Gl. diaphanum is a membranous layer, which is lacking in the spore wall of the species discussed here.
Considering Gl. pubescens, only its penultimate laminate spore wall layer links this fungus with Gl. proliferum, in which the laminate layer is the innermost structure in its spore wall, however (Blaszkowski 2003). Spores of the latter fungus do not possess the permanent, unit outermost spore wall layer and the flexible innermost one of the former fungus .
Although the outermost spore wall layers of Gl. proliferum and Par. occultum are of the type of sloughing layers, they differ in biochemical properties. In Gl. proliferum, it stains intensively in Melzer's reagent, whereas it is nonreactive in Par. occultum in this reagent (Morton 2002; Morton and Redecker 2001). Additionally, in Par. occultum, the other two layers of its spore wall are laminate (Morton and Redecker 2001). In contrast, only one such a layer occurs in the spore wall of Gl. proliferum.
As mentioned above, the spore wall of Pac. franciscana also consists of three layers, of which only the middle laminate one phenotypically shares this fungus with Gl. proliferum (Blaszkowski 2003; Oehl and Sieverding 2004). The other two spore wall layers of the former fungus are permanent and do not degenerate with age, as layers 1 and 2 of the spore wall of the latter species do. However, the most important taxonomically difference separating these fungi is that Pac. franciscana still differentiates a 3-layered inner germination wall, which does not do Gl. proliferum.
Compared with the 3-layered spore wall of Gl. proliferum, the spore wall of Gl. cerebriforme, Gl. minutum, and Par. laccatum comprises only two layers (McGee 1986; Blaszkowski 2003; Blaszkowski et al. 2000; Renker et al. 2007). Additionally, differences between these species regard the spatial position of the structural laminate layer of their spores, the compactness of this layer, and the phenotypic and biochemical properties of the second layer. In spores of Gl. proliferum and Gl. minutum, the laminate layer is the innermost component of this wall, and in Gl. cerebriforme it forms the spore surface. While the laminae of this layer tightly adhere to each other in vigorously crushed spores of Gl. cerebriforme, Gl. minutum and Gl. proliferum, the laminate wall layer of spores of Par. laccatum easily stratifies in even slightly crushed spores. The second spore wall layers of the species compared here are a flexible inner layer (Gl. cerebriforme), a flexible, permanent outer one (Gl. minutum), and an evanescent outer layer (Par. laccatum), which are lacking in the spore wall of Gl. proliferum. Moreover, except for the outermost mucilaginous layer of the Gl. proliferum spore wall staining in Melzer's reagent, none of the spore wall layers of the other species compared here reacts in this reagent.
As mentioned at the beginning of this section, Gl. proliferum originally characterized by Declerck et al. (2000) and that sent to the author of this book distinguished its colourless spores. Meanwhile, microphotographs of this fungus provided by Dr. C. Walker, Royal Botanic Garden Edinburgh, UK, showed it to form pale yellow spores too (Blaszkowski, pers. comm.). Thus, Gl. proliferum may also be confused with species of the Glomeromycota whose glomoid spores darken from hyaline to pale yellow. These are Diversispora spurca (C.M. Pfeiff., C. Walker & Bloss) C. Walker & Schuessler, Gl. albidum C. Walker & L.H. Rhodes, Gl. fasciculatum (Thaxt.) Gerd. & Trappe emend. C. Walker & Koske, Gl. gibbosum Blaszk., Gl. intraradices N.C. Schenck & G.S. Sm., Gl. nanolumen Koske & Gemma, Gl. segmentatum Trappe, Spooner & M.H. Ivory, Gl. vesiculiferum (Thaxt.) Gerd. & Trappe, Gl. viscosum Nicol., Gl. walkeri Blaszk. & C. Renker, and Pac. brasilianum (Spain & J. Miranda) J.B. Morton & D. Redecker. Except for Gl. intraradices found to be a fungus also most closely related molecularly to Gl. proliferum (Declerck et al. 2000), the other species are easy to separate from it because of differences in (1) size of their spores (D. spurca, Gl. albidum, Gl. fasciculatum, Gl. gibbosum, Gl. viscosum, Par. brasilianum), (2) the spore wall structure and phenotypic properties of its components (D. spurca, Gl. albidum, Gl. gibbosum, Gl. nanolumen, Gl. segmentatum, Gl. vesiculiferum, Gl. viscosum, Gl. walkeri), and (3) the reactivity of the spore wall layers in Melzer's reagent (D. spurca, Gl. albidum, Gl. fasciculatum, Gl. gibbosum, Gl. nanolumen, Gl. segmentatum, Gl. viscosum; Blaszkowski 1997, 2003; Blaszkowski et al. 2006; Gerdemann and Trappe 1974; Koske and Gemma 1989; Trappe 1979; Morton 2002; Morton and Redecker 2001; Walker and Rhodes 1981; Walker et al. 1995).
Pale yellow spores of Gl. proliferum may be difficult to distinguish from mature small-spored strains of Gl. intraradices when observed under both low and high microscope magnifications. In both fungi, they form in loose aggregates, frequently inside roots, and overlap in colour and size (Blaszkowski 2003). The wall structure of spores and both the phenotypic and biochemical properties of its components also are almost identical (Blaszkowski 2003; Morton 2002; Stürmer and Morton 1997). The outer two spore wall layers of Gl. proliferum have the same thickness and are of the same type of layers as those of Gl. intraradices. Moreover, the outermost spore wall layer in Gl. proliferum and Gl. intraradices stains intensively and similarly in Melzer's reagent (Blaszkowski 2003). The main differences markedly separating these fungi hide in the characters of the third spore wall layer and the subtending hypha. Although the third spore wall layer of the two fungi discussed here consists of adherent laminae (sublayers), the laminae of the wall of Gl. proliferum spores are much thinner (<0.5 µm thick; Blaszkowski, pers. observ.) and tightly adhere to each other in even vigorously crushed spores, whereas those of the Gl. intraradices spore wall are ca. 0.5-1.0 µm thick and usually stratify in even intact spores (Blaszkowski 2003). Additionally, the spore wall of the former fungus is much thinner (1.2-2.2 µm thick; Blaszkowski 2003). Finally, compared with the subtending hypha of spores of Gl. proliferum, that of Gl. intraradices spores is wider by ca. 50% (8.8-11.2 µm wide vs. 12.7-18.4 µm wide; Blaszkowski 2003).
However, even mean in size globose spores of Gl. intraradices (92.0 µm diam; Blaszkowski 2003) are markedly larger than the largest spores of Gl. proliferum (76.0 µm diam; Declerck et al. 2000).
REFERENCES
Blaszkowski J. 1988. Three new vesicular-arbuscular mycorrhizal fungi (Endogonaceae) from Poland. Bull. Pol. Acad. Sci. Biol. Sci. 36, 271-275.
Blaszkowski J. 1997. Glomus gibbosum, a new species from Poland. Mycologia 89, 339-345.
Blaszkowski J. 2003. Arbuscular mycorrhizal fungi (Glomeromycota), Endogone and Complexipes species deposited in the Department of Plant Pathology, University of Agriculture in Szczecin, Poland. http://www.agro.ar.szczecin.pl/~jblaszkowski/.
Blaszkowski J., Renker C., Buscot F. 2006. Glomus drummondii and G. walkeri, two new species of arbuscular mycorrhizal fungi (Glomeromycota). Mycol. Res. 110, 555-566.
Blaszkowski J., Tadych M., Madej M. 2000. Glomus minutum, a new species in Glomales (Zygomycetes) from Poland. Mycotaxon 76, 187-195.
Declerck S., Cranenbrouck S., Dalpé Y., Séguin S., Grandmougin-Ferjani A., Fontaine J., Sancholle M. 2000. Glomus proliferum sp. nov.: a description based on morphological, biochemical, molecular and monoxenic cultivation data. Mycologia 92, 1178-1187.
Gerdemann J. W., Trappe J. M. 1974. The Endogonaceae in the Pacific Northwest. Myc. Memoir 5, 1-76.
Koske R. E., Gemma J. N. 1989. Glomus nanolumen (Endogonaceae), a new species from Hawaii. Mycologia 81, 935-938.
McGee P. A. 1986. Further sporocarpic species of Glomus (Endogonaceae) from South Australia. Trans. Brit. Mycol. Soc. 87, 123-129.
Morton J. B. 2002. International Culture Collection of (Vesicular) Arbuscular Mycorrhizal Fungi. West Virginia University. http://www.invam.caf.wvu.edu/.
Morton J. B., Redecker D. 2001. Two families of Glomales, Archaeosporaceae and Paraglomaceae, with two new genera Archaeospora and Paraglomus, based on concordant molecular and morphological characters. Mycologia 93, 181-195.
Oehl F., Sieverding E. 2004. Pacispora, a new vesicular arbuscular mycorrhizal fungal genus in the Glomeromycetes. J. Appl. Bot. 78, 72-82.
Renker C, Blaszkowski J, Buscot F 2007. Paraglomus laccatum comb. nov. - a new member of Paraglomeraceae (Glomeromycota). Nova Hedwigia 84 (3-4), 395-407.
Schwarzott D., Walker C., Schüßler A. 2001. Glomus, the largest genus of the arbuscular mycorrhizal fungi (Glomales) is nonmonophyletic. Mol. Phyl. Evol. 21, 190-197.
Stürmer S. L., Morton J. B. 1997. Developmental patterns defining morphological characters in spores of four species in Glomus. Mycologia 89, 72-81.
Trappe J. M. 1979. Glomus segmentatum sp. nov. Trans. Brit. Mycol. Soc. 73, 362-363.
Walker C., M. Giovannetti L. Avio A. S. Citernesi, Nicolson T. H. 1995. A new fungal species forming arbuscular mycorrhizas: Glomus viscosum. Mycol. Res. 99, 1500-1506.
Walker C., Rhodes L. H. 1981. Glomus albidus: a new species in the Endogonaceae. Mycotaxon 12, 509-514.