LIFE CYCLE, SIGNIFICANCE, AND STRUCTURES OF ARBUSCULAR MYCORRHIZAE
A symbiotic association of a fungus and roots has been discovered in Monotropa hypopitys L. by Franciszek Kamienski (Kamienski 1881), a Polish mycologist. Later, Frank (1885) coined the term “mycorrhiza” to the association.
Professor Franciszek Kamienski
(1851-1912)
Arbuscular mycorrhizae form or are considered to form fungi of thirteen of the fourteen genera of the phylum Glomeromycota (Blaszkowski 2003; Schüßler at al. 2001). The fourteen genus of the phylum, Geosiphon contains only G. pyriformis, which produces endocytosymbioses with photoautotrophic prokaryotes (Schüßler 2002; Schüßler and Kluge 2001).
Arbuscular mycorrhizal fungi are obligate biotrophs feeding only on the products of photosynthesis of their alive plant hosts. Generally, the fungi are not specialized to their potential hosts, although some plant species more favour the development of these fungi than others (Blaszkowski 1993; Smith and Read 1997). The fungi belong to the most commonly occurring soil microorganisms of the world and are associated with at last 80% of plants of the Earth (Gianinazzi and Gianinazzi-Pearson 1986), including angiosperms, gymnosperms and pteridophytes having roots, as well as the gametophytes of some mosses, lycopods, and Psilotalus, which do not have true roots (Smith and Read 1997).
Literature data indicate that arbuscular mycorrhizal fungi increase the root absorptive area and hence the plant nutrition (Bieleski 1973), influence succession of plant communities (Janos 1980), their competitiveness (Allen and Allen 1984; Fitter 1977) and phenology (Allen and Allen 1986), equalize the level of nutrition of co-existing plants by formation of hyphal bridges transferring nutrients between them (Newman 1988), and improve soil structure through binding sand grains into aggregates by extraradical hyphae (Koske et al. 1975; Sutton and Sheppard 1976). Additionally, arbuscular mycorrhizal fungi increased the tolerance of plants to heavy metals (Dehn and Schüepp 1989; Griffioen and Ernst 1989), water stresses (Stahl and Smith 1984), as well as pathogenic fungi and nematodes (Schönbeck 1978). The requirement of arbuscular fungi for up to 20% of host photosynthate for establishment and maintenance is well accepted (Graham 2000; Jakobsen and Rosedahl 1990).
Arbuscular mycorrhizae consist of intra- and extraradical structures. The intraradical structures are arbuscules, vesicles, and intraradical hyphae. The extraradical structures are extraradical hyphae, spores, and auxiliary cells, the latter are formed only by members of the genera Gigaspora, Pacispora, and Scutellospora.
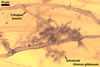
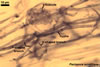
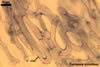
Haustorium-like arbuscules are the main sites of nutrient exchange between a plant host and a fungus (Gianinazzi et al. 1979). They are formed within the cells of the inner root cortex (Mosse 1973) and are indicators of active mycorrhizae.
In roots
of Plantago lanceolata |
In roots of Zea mays |
||
Arbuscules differ in morphology, depending on the generic affiliation of the arbuscular fungal species (Morton 2000). Fungi of the genera Acaulospora, Archaeospora, Ambispora, Diversispora, Entrophospora, Glomus, Intraspora, Kuklospora, Pacispora, and Paraglomus produce arbuscules with cylindrical or slightly flared, narrow trunks, whose branches progressively taper in width towards tips. Arbuscules of members of the genera Gigaspora and Scutellospora generally have swollen trunks with branches tapering abruptly at tips. The characters of mycorrhizae of Otospora bareai, the only member of the genus Otospora, have not been recognized to date (Palenzuela et al. 2008).
In roots of P. lanceolata |
In roots of Z. mays |
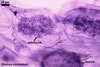
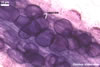
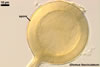
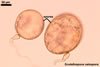
Globose or ovoid, thin-walled vesicles are storage organs filled with lipids and glycolipids (Mosse 1981). They origin by an intercalary or terminal swelling of a mycorrhizal intraradical hypha of an arbuscular fungus. Vesicles may be inter- or intracellular and may be found in both the inner and the outer layers of the cortical parenchyma. In Glomus spp., vesicles generally are ellipsoid, whereas those of Acaulospora, Entrophospora, and Kuklospora highly vary in shape and frequently have knobs and concavities on their surface (Morton 2000). Not all Glomus spp. form vesicles (Morton and Redecker 2001). They are never produced by members of the genera Gigaspora and Scutellospora. Members of the genera Archaeospora, Intraspora, and Paraglomus rarely produce vesicles or do not form them at al.
In roots of Plantago
lanceolata |
||
Arbuscular mycorrhizae also differ in the degree of evenness of distribution along roots and the intensity of staining. The distribution of mycorrhizal structures of members of the genera Ambispora, Archaeospora, Acaulospora, Diversispora, Entrophospora, Intraspora, Kuklospora, and Paraglomus is patchy, whereas that of mycorrhizae of the genera Gigaspora, Glomus, Pacispora, and Scutellospora usually is continuous. The intensity of staining of mycorrhizae of fungi of the genera Ambispora, Archaeospora, Diversispora, Intraspora, and Paraglomus is very faint to faint, those of Acaulospora, Entrophospora, and Kuklospora faint to moderate, those of Glomus dark, and those of Gigaspora, Pacispora, and Scutellospora very dark (Blaszkowski, pers. observ.; Morton and Redecker 2001; Sieverding and Oehl 2006).
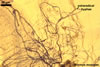 |
 |
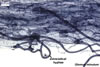 |
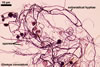
Extraradical hyphae significantly increase the absorptive area of roots (Bieleski 1973), form hyphal bridges transferring nutrients between co-occurring plants (Newman 1988), and bind sand grains into aggregates (Koske and Polson 1984). They also are important fungal propagules colonizing plant roots (Jasper et al. 1989, 1991).
Auxiliary cells are swollen structures produced terminally by extraradical hyphae of only Gigaspora, Pacispora, and Scutellospora spp. The cells are spiny in Gigaspora spp., and those of species of the genera Pacispora and Scutellospora are smooth or knobby (Blaszkowski 2003; Morton 2002).
The patterns of spore development differ significantly in the genera of the phylum Glomeromycota recognized. They along with properties of mycorrhizae formed by members of the genera are characterized below.
• Development of spores and properties of mycorrhizae of fungi of the genus Acaulospora
• Development of spores and properties of mycorrhizae of fungi of the genus Archaeospora
• Development of spores and properties of mycorrhizae of fungi of the genus Ambispora
• Development of spores and properties of mycorrhizae of fungi of the genus Diversispora
• Development of spores and properties of mycorrhizae of fungi of the genus Entrophospora
• Development of spores and properties of mycorrhizae of fungi of the genus Gigaspora
• Development of spores and properties of mycorrhizae of fungi of the genus Glomus
• Development of spores and properties of mycorrhizae of fungi of the genus Intraspora
• Development of spores and properties of mycorrhizae of fungi of the genus Kuklospora
• Development of spores and properties of mycorrhizae of fungi of the genus Pacispora
• Development of spores
and properties of mycorrhizae of fungi of the genus Paraglomus
• Development of spores and properties of mycorrhizae of fungi of the genus Scutellospora
REFERENCES
Allen E. B., Allen M. F. 1984. Competition between plants of different successional stages: mycorrhizae as regulators. Can. J. Bot. 62, 2625-2629.
Allen E. B., Allen M. F. 1986. Water relations of xeric grasses in the field: interactions of mycorrhizae and competition. New Phytol. 104, 559-571.
Berch S. M., Fortin J. A. 1983. Lectotypification of Glomus macrocarpum and proposal of new combinations: Glomus australe, Glomus versiforme, and Glomus tenebrosum (Endogonaceae). Can. J. Bot. 61, 2608-2617.
Bieleski R. L. 1973. Phosphate pools, phosphate transport and phosphate availability. Ann. Rev. Plant Physiol. 24, 225-252.
Blaszkowski J. 1993. Comparative studies of the occurrence of arbuscular fungi and mycorrhizae (Glomales) in cultivated and uncultivated soils of Poland. Acta Mycol. 28, 93-140.
Blaszkowski J. 2003. Arbuscular mycorrhizal fungi (Glomeromycota), Endogone, and Complexipes species deposited in the Department of Plant Pathology, University of Agriculture in Szczecin, Poland.http://www.agro.ar.szczecin.pl/~jblaszkowski/.
Blaszkowski J., Madej T., Tadych M. 1998. Entrophospora baltica sp. nov. and Glomus fuegianum, two species in the Glomales from Poland. Mycotaxon 68, 165-184.
Daft M. J., El-Giahmi A. A. 1978. Effect of arbuscular mycorrhiza on plant growth. VIII. Effects of defoliation and light on selected hosts. New Phytol. 80, 365-372.
Dehn B., Schüepp H. 1989. Influence of VA mycorrhizae on the uptake and distribution of heavy metals in plants. Agric. Ecosys. Environ. 29, 79-83.
Fitter A. H. 1977. Influence of mycorrhizal infection on competition for phosphorus and potassium by two grasses. New Phytol. 79, 119-125.
Frank A. B. 1885. Über die auf Wurzelsymbiose beruchende Ernarung gewisser Baume durch unterirdische Pilze. Ber. Deutch Bot. Gessell. 3, 128-145.
Gemma J. N., Koske R. E., Carreiro M. 1989. Seasonal dynamics of selected species of VA mycorrhizal fungi in a sand dune. Mycol. Res. 92, 317-321.
Gianinazzi S., Gianinazzi-Pearson V. 1986. Progress and headaches in endomycorrhiza biotechnology. Symbiosis 2, 139-149.
Gianinazzi S., Gianinazzi-Pearson V., Dexheimer J. 1979. Enzymatic studies on the metabolism of vesicular-arbuscular mycorrhiza. II. Ultrastructural location of acid and alkaline phosphatase in onion roots infected by Glomus mosseae Nicol. & Gerd. New Phytol. 82, 127-132.
Giovannetti M. 1985. Seasonal variations of vesicular-arbuscular mycorrhizas and Endogonaceous spores in a maritime sand dunes. Trans. Br. Mycol. Soc. 84, 679-684.
Graham J. H. 2000. Assessing costs of arbuscular mycorrhizal symbiosis in agroecosystems. In: Podila G. K., Douds D. D. Jr. (eds.). Current Advances in Mycorrhizal Research. APS Press, St. Paul, MN., 127-140.
Griffioen W. A. J., Ernst W. H. O. 1989. The role of VA mycorrhiza in the heavy metal tolerance of Agrostis capillaris L. Agric. Ecosys. Environm. 29, 173-177.
Hayman D. S. 1970. Endogone spore numbers in soil and vesicular-arbuscular mycorrhiza in wheat as influenced by season and soil treatment. Trans. Br. Mycol. Soc. 554, 53-63.
Hetrick B. A. D., Bloom J. 1986. The influence of host plant on production and colonization ability of vesicular-arbuscular mycorrhizal spores. Mycologia 78, 32-36.
Jacobsen I., Rosendahl L. 1990. Carbon flow into soil and external hyphae from rotos of mycorrhizal cucumber plants. New Phytol. 115, 77-83.
Janos D.P. 1980. Mycorrhizae influence tropical succession. Biotropica 12, 56-64.
Jasper D. A., Abbott L. K., Robson A. D. 1989. Soil disturbance reduces the infectivity of external hyphae of vesicular- arbuscular mycorrhizal fungi. New Phytol. 112, 93-99.
Jasper D. A., Robson A. D., Abbott L. K. 1991. The effect of soil disturbance on vesicular-arbuscular mycorrhizal fungi in soils from different vegetation types. New Phytol. 118, 471-476.
Kamienski F. 1881. Die Vegetationsorgane der Monotropa hypopitys L. Bot. Zeitschr. 39, 225-234.
Koske R. E. 1985. Glomus aggregatum emended: A distinct taxon in the Glomus fasciculatum complex. Mycologia 77, 619-630.
Koske R. E., Sutton J.C., Sheppard B. R. 1975. Ecology of Endogone in Lake Huron sand dunes. Can. J. Bot. 53, 87-93.
Koske R. E., Polson W. R., 1984. Are VA mycorrhizae required for sand stabilization ? Bioscience 34, 420-424.
Morton J. B. 1993. Problems and solutions for the integration of glomalean taxonomy, systematic biology, and the study of endomycorrhizal phenomena. Mycorrhiza 2, 97-109.
Morton J. B. 2002. International Culture Collection of Arbuscular and Vesicular-Arbuscular Mycorrhizal Fungi. West Virginia University. http://www.invam.caf.wvu.edu/.
Morton J. B., Redecker D. 2001. Two families of Glomales, Archaeosporaceae and Paraglomaceae, with two new genera Archaeospora and Paraglomus, based on concordant molecular and morphological characters. Mycologia 93, 181-195.
Mosse B. 1973. Plant growth response to vesicular-arbuscular mycorrhizae. X. response of Stylosanthes and maize to inoculation in unsterile soils. new Phytol. 78, 277-288.
Mosse B. 1981. Vesicular-Arbuscular Mycorrhiza Research for Tropical Agriculture. Hawaii Institute of Tropical Agriculture and Human resources, Univ. of Hawaii.
Newman E. I. 1988. Mycorrhizal links between plants: their functioning and ecological significance. Adv. Ecol. Res. 18, 243-270.
Palenzuela J., Ferrol N., Boller T., Azcón-Aguilar C., Oehl F. 2008. Otospora bareai, a new fungal species in the Glomeromycetes from a dolomitic shrubland in the Natural Park of Sierra de Baza (Granada, Spain). Mycologia (in press).
Schönbeck F. 1978. Einfluss der endotrophen Mykorrhiza auf die Krankheitsresistenz höherer Pflanzen. Z. PflKrank. PflSchutz 85, 191-196.
Schüßler A. 2002. Molecular phylogeny, taxonomy, and evolution of Geosiphon pyriformis and arbuscular mycorrhizal fungi. Plant and Soil 244, 75-83.
Schüßler A., Kluge M. 2001. Geosiphon pyriforme, an endocytosymbiosis between fungus and cyanobacteria, and its meaning as a model system for AM research. In: Hock B. (ed.). The Mycota. Vol. IX. Fungal Associations, 151-161. Springer, Berlin.
Schüßler A. Schwarzott D., Walker C. 2001. A new fungal phylum, the Glomeromycota: pylogeny and evolution. Myc. Res. 105, 1413-1421.
Sieverding E., Oehl F. 2006. Revision of Entrophospora and description of Kuklospora and Intraspora, two new genera in the arbuscular mycorrhizal Glomeromycetes. J. Appl. Bot. Food Qual. 80, 69-81.
Smith S. E., Read D. J. 1997. Mycorhizal symbiosis. Academic Press. Harcourt Brace & Company, Publishers. San Diego, London, New York, Boston, Sydney, Tokyo, Toronto.
Stahl P. O., Smith W. K. 1984. Effects of different geographic isolates of Glomus on the water relations of Agropyron smithii. Mycologia 76, 261-267.
Sutton J. C., Sheppard B. R. 1976. Aggregation of sand-dune soil by endomycorrhizal fungi. Can. J. Bot. 54, 326-333.
Tommerup I. C., Sivasithamparam K. 1990. Zygospores and asexual spores of Gigaspora decipiens, an arbuscular mycorrhizal fungus. Mycol. Res. 94, 897-900.